Handout
advertisement

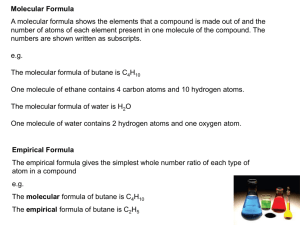
Chemistry 11 Unit 5: The Mole Concept Name:____________________ Date:______________Bl:____ Learning Goal: To understand the relationship between mass, number and type of particles, volume, and concentration for any chemical. Concept 6: Finding the Molecular Formula of a Pure Substance Empirical vs. Molecular _______________ is the smallest _______________ of a molecule while _______________ is always some multiple of the empirical formula to reflect the actual compound structure. So… Which of the following are empirical formulae? Which are molecular formulae? Pure Substance Empirical or Molecular? C12H24 C100H200 CH2 C26H52 C3H6 Finding the molecular formula… ☼ A 5-step process Ex) 2.10181 x 1023 molecules of a compound is composed of 16.66g of carbon and 3.49g of hydrogen. Find the molecular formula. Step 1: Find the empirical formula (this is a 4-step process… see notes from last class) Hint: pg. 93! Step 2: Calculate the empirical molar mass. Step 3: Calculate the molecular molar mass (if not given). Step 4: Find the multiple. Use: N = molecular molar mass empirical molar mass Where N = the multiple Step 5: Use the multiple to find the molecular formula. molecular formula = N × (empirical formula) Now: Try #47-55 on page 95. Next Class: Checkpoint #2 – Concepts 1, 2, 3, 4, 5, 6