Presentation to HESI Members in Japan
advertisement

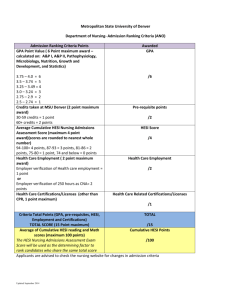
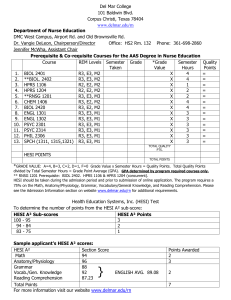
HESI: Health and Environmental Sciences Institute Syril D Pettit, MEM Executive Director February 25, 2015 Member Meeting HESI Washington, DC USA www.hesiglobal.org ILSI Health and Environmental Sciences Institute HESI Mission Create science-based solutions for a sustainable, healthier world. Accurate and Efficient Chemical Risk Assessment Safe and Effective Medicines Environmental Quality and Sustainability Food Safety Protecting sensitive populations Sustaining critical environments Supporting ecological and human safety of essential food resources Promoting Discovery Predicting and Protecting Against Adverse Effects from Chronic Exposures The HESI Model: Bridging Research to Application Academic & basic research sector SAFETY & INNOVATION FOR HUMAN & ENVIRONMENTAL HEALTH Patient Advocates, Foundations & NGOs Industry R&D Government Research & Regulation 90 University & Research Centers From 14 Countries 47 Government Agencies 66 Corporate Sponsors Across multiple sectors From 12 Countries 14 Scientific Committees >70 Distinct Projects Impact via Quality Science HESI achieves its mission via… Scientific Research Millions in in-kind research annually Publication Communication & Translation Tools Training Novel programs with interdisciplinary and cross-sector focus Platforms for Interaction Active public-private partnership HESI: A Key Contributor to Global Safety Research In 2014/2015…. Two OECD supportive research projects Pig-A genotoxicity mutation assay Fish hepatic biotransformation assay – bioaccumulation HESI research part of active guideline discussions with ICH Cardiac paradigms (CIPA project) Developmental toxicity (2nd Species Project) We know the model works… HESI’s scientific programs and publications have.. Influenced their approach to safety or risk assessment decision-making; 70% Influenced their level of confidence in the use of particular technologies, markers, endpoints, or analysis approaches; 80% Directly and positively impacted safety of patients and the environment Organization Strong & Growing Increasing public and private sector involvement 65 History of Success Creating frameworks to integrate data and decision-making Prioritize risks, Protect ecosystems and their inhabitants Integrating Alternatives to Animal Testing for Ecotox Globally recognized HESI roadmaps to guide integration of data and decisions. Mode of Action Risk / Safety In vivo In vitro Toxicity Innovating Chemical Risk Assessment Matrix QSAR/ TTC Biomonitoring Probabilistic Deterministic Minimal Info Assessing Adverse vs Adaptive Transitions in Toxicity Pathways Exposure Problem Formulation Conclude Enhancing AgChem Safety A spotlight on one of many… US EPA Scientific and Technological Basis for OECD Guideline for Achievement Award Testing of Chemicals (443): (Honorable Mention) Extended One-Generation Reproductive Toxicity Study UK National Center for the Canine study requirement Replacement, Refinement, and dropped in EPA Pesticide Reduction of Animals in Research guidelines; “Highly Commended Prize” Increased use of ADME to enhance dose selection Impact cited in 2 National Academy reports Enhancing AgChem Safety Informing discovery & decision-making with new technologies Toxicogenomics • First large scale TGx experimental program, first public array/tox dbase for Risk • Led to adoption of data standards, Assessment genomic biomarkers • Resource for strengths & limitations of TgX use for safety Transgenic Models for Cancer Risk Assessment • $33M collaborative effort • Critical data on predictivity of available transgenic models • Data underpins current guidelines on alternatives to 2 year mouse bioassay • Improved prediction of safety Translating from animal to human, and back to improve predictive safety HESI Approach to Biomarkers • Consensus on Safety or Translational Need • Experimental Data • Analysis & Publication Nonclinical cTn serum assays • Integration of Data & Context of Use Nonclinical Inhibin Assays Urinary Renal Protein Biomarkers MicroRNAs as translation tox markers 2015: PROGRAMS TO IMPACT SCIENCE AND HEALTH Chemical Safety Evaluation Predictive models Risk assessment methodologies Sustainability Capacity Building & Education Predictive Models Zebrafish & multigenerational epigenetics Bioaccumulation: In vitro method, hepatic clearance in trout Utility of 2nd Species for assessing developmental toxicity Pig-A assay for genotoxicity Risk Assessment Methodologies RISK21 AOP and Ecotox Sustainability Capacity Building & Education Scientist from the following organization’s collaborate with HESI on Chemical Risk Assessment research…. and many more! SAFE AND EFFECTIVE MEDICINES ‘Crossing over the valley of death’ FasterCures. 2010. http://www.fastercures.org/reports/view/3 Underfunded? Or inefficient? FasterCures. 2010. http://www.fastercures.org/reports/view/3 Region of Opportunity Patient Experience Contemporary Drivers Enhance drug discovery, don’t compromise safety Support Cancer Patient Survival & Quality of Life Prevent Another TGN 1412 Ensure new therapeutic modes are safe for sensitive populations Enhance drug discovery, don’t compromise safety What It Will Do: - Nature Review: Drug Discovery. Aug 2013 - Prevent early attrition due to hERG liabilities Provide a more complete assessment of proarrhythmic risk Obviate need for a TQT study and improve efficiency Potential to re-label drugs with risk warnings Standardize in vitro & in silico assays; establish best practices for stem-cell derived cardiomyocytes Likely lead to revision of S7B, E14 guidelines Comprehensive In Vitro Proarrhythmia Assay: Three Core Pillars Drug Effects on Multiple Human Cardiac Currents In Silico In Vitro Effects Reconstruction Human StemHuman Cell Derived Ventricular Ventricular Cellular Myocytes Electrophysiology Evaluation of Clinical Drugs for Proarrhythmic TdP Liability High Risk Intermediate Risk Low Risk Preclinical ECG & Phase 1 ECG Studies: Complementary Data Prevent Another TGN 1412 HESI STUDY RESULTS: Significant variability revealed (controls, assay selection, design, situational use, interpretation) Identified opportunities to improve forward prediction with collaborative approach HESI MOVING FORWARD Centralized repository for positive/ negative controls for CRA Prospective study across labs w consistent protocols, controls: outcome to help ID sources of variability, build best practices, guide interpretation. Ensure new therapeutic modes are safe for sensitive populations FC-Fusion Proteins (Biopharmaceuticals) a significant new area of therapeutic development - FC domain enhances half-life - Biopharmaceuticals with Fc region from IgG are known to cross placenta - How to assess safety for women of childbearing age? Support Cancer Patient Survival & Quality of Life Simple numbers…terrible cost • 7 fold higher risk of cardiac mortality www.fredhutch.org HESI’s is innovating the field with support for: - modeling -translational safety signals or monitoring -interdisciplinary platforms Scientist from the following organizations collaborate with HESI on Drug Safety research…. and many more! The HESI Model: Bridging Research to Application Academic & basic research sector SAFETY & INNOVATION FOR HUMAN & ENVIRONMENTAL HEALTH Patient Advocates, Foundations & NGOs Industry R&D Government Research & Regulation CREATING NEW COMMUNITIES OF PRACTICE TO ENHANCE RELEVANCE & EFFICIENCY IMPLEMENTING FIT FOR PURPOSE SCIENCE TO MEET PATIENT & ENVIRONMENTAL NEEDS POOLING RESOURCES TO EXPEDITE EFFORTS, DIVERSIFY EXPERTISE-BASE Bridging Innovative Research and Application to Enhance Safety MOVING OUTCOMES INTO PUBLIC DOMAIN TO BENEFIT PUBLIC HEALTH AND ENVIRONMENT COMMUNITY AT LARGE HESI Strategic Plan Renewal 2015 Current plan expires in 2015 Process for renewal launched in fall 2014, Tecker & Associates hired PATH FORWARD January 2015 – ‘Environmental Scan’ & Values Statement N First Quarter – Survey input from members, EIC, ORs & selected stakeholders June 10-12, 2015 – Extended strategic plan strategy session at June 2015 Annual Meeting (all invited) 4Q 2015 – Finalization of the 2016-2020 Strategic Plan January 2016 – Board Vote to Approval 2016+ Strategy • • • N • • • • • Some topics identified in January 2015 Shifts in resource availability Greater emphasis on technology for evaluation and every day tasks New opportunities for expertise (crowd sourcing, developing regions, etc.) Globalization and global economy Shifting health care priorities and concerns (epidemics, aging population, personalized medicine, etc.) Digital communication – speed and transfer of information and news New approaches to how science research is conducted and communicated New regulatory environments (animal welfare, environmental concerns, risk aversion, harmonization, public involvement, etc.) Your Thoughts? N • • NEW HESI PROJECTS Emerging Issues Committee Update – February 2015 New Subcommittee: Framework for Intelligent Non-Animal Alternative Methods for Safety Assessment Selection and Initiation: • • Selected by EIC in fall 2014. First meeting of Cmte in January 2015. Leadership: Prof. Alan Boobis Dr. Craig Rowlands Imperial College London Dow Chemical Company Dr. Natalie Burden NC3Rs Dr. Beatrize Silva Lima University of Lisbon Dr. Matt Hurtt Pfizer Dr. Norbert Kaminski Michigan State University Dr. Suzanne Fitzpatrick US FDA Staff: Dr. Stan Parish (HESI Scientific Program Manager) Objective: Develop a set of consistent, internationally relevant criteria against which the reliability and fitness-for-purpose of new non-animal methods or approaches are assessed. EI Proposal Solicitation: Timeline TIMELINE: 15 December 2014: Proposals due to HESI. 10 February 2015: Submitted proposals undergo detailed review by EIC. March 2015: Submitters are contacted by HESI staff with decisions about each proposal. June 2015: Selected proposals are presented at the HESI Annual Meeting. Summer 2015: Proposals presented at the HESI Annual Meeting are distributed for prioritization and voting by the HESI constituency. Fall 2015: The EIC selects one or two proposals for action. Topics for Voting in June 2015 • Nonclinical efficacy and safety studies in support of neonatal pediatric therapeutic use and development • Models/tests to assess stem cells as therapeutic agents – safety and mode of action and efficacy. • Frameworks for assessing the protectiveness and predictability of human health risk assessments • Transforming exposure science through emerging technologies and big data to improve predictive exposure capabilities. Other New projects ECO ASSESSMENT Development of ecotoxicological TTC (ecoTTC) Effluent testing for ecotoxicity BIOMARKERS • Cardiac biomarkers after exposure to doxorubicin/diclofenac in the Zucker Diabetic Fatty rat model • Biomarkers of prolactin (evaluating stress vs. chemical-induced responses) IN VITRO APPROACHES • Testing developmental toxicants in alternative in vitro assays. NEW TECHNOLOGIES • Workshop: Fetal imaging in regulatory toxicity testing HESI ANNUAL MEETING JUNE 9-12, 2015 WASHINGTON, DC SAVE THE DATE!!!! HESI: Health and Environmental Sciences Institute Syril D Pettit, MEM Executive Director spettit@hesiglobal.org Ayako Takei, MPH HESI Science Advisor in Japan atakei@hesiglobal.org ILSI Health and Environmental Sciences Institute BACKUPS HESI Staff Syril Pettit, MEM, Executive Director Nancy Doerrer, MS, Associate Director Cynthia Nobles, Branch Administrator Michelle Embry, PhD Senior Scientific Program Manager Connie Chen, MPH, PhD Scientific Program Manager Stanley Parish, PhD Scientific Program Manager Brianna Farr, Science Program Associate Raegan O’Lone, PhD Senior Scientific Program Manager Jennifer Tanir, PhD Jennifer Pierson, MPH Scientific Program Manager Scientific Program Manager Oscar Bermudez Science Program Associate • http://www.monticello.org/site/research-and-collections/historic-landscape-institute Science never appears so beautiful as when applied to the uses of human life. Thomas Jefferson, 1798. Charlottesville, Virginia.