Solutions - AaronFreeman
advertisement

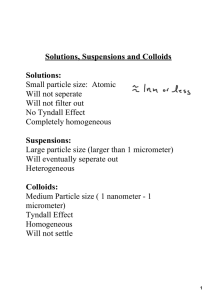
Solutions Parts of a Solution Molarity, Molality, Mole Fraction, Dilution Types of Mixtures • Mixtures : a blend of two or more kinds of matter, each of which retains its own properties and characteristics • Homogeneous vs. Heterogeneous Mixtures • Homo = same Hetero = varied Solutions • Solution : homogeneous mixture of two or more substances in a single phase Parts of a Solution : • Solute – dissolved particles • Solvent – dissolving medium • Aqueous = capable of being dissolved EX. Salt, sugar EX. Water, alcohol Concentrated vs. Dilute What is Molarity? • Moles of solute per volume of solution • Equation is GIVEN on Reference Table! Moles of solute per volume of total SOLUTION Mol of solute Liters of solution Moles of solute per mass of SOLVENT Mol of solute Kg of solvent mol L mol kg M m What is molality? • Moles of solute per mass of solvent • Equation is NOT GIVEN on Reference Table! Molarity Examples • EXAMPLE : 3.50 L of solution contains 90.0 g of NaCl. What is the molarity of the solution? • EXAMPLE : 0.800 L of a .500 M HCl solution. How many moles of HCl does this solution contain? Molality Examples • EXAMPLE : Solution was prepared by dissolving 17.1 g of sucrose (C12H22O11) in 125 g of water. Find the molal concentration of solution. • EXAMPLE : A solution iodine (I2) in carbon tetrachloride (CCl4) is used when iodine is needed for certain chemical tests. How much iodine must be added to prepare a 0.480 m solution of iodine in CCl4 if 100.00 g of CCl4 is used? What is mole fraction? • MOLE FRACTION () = • EXAMPLE : What is the mole fraction of xenon in a mixture that contains 0.584 g of xenon 86.40 g of argon and 3.62 g of neon ? How many solute parts? Dilutions 13 13 What’s the only difference? How can you dilute a concentrated solution? • DILUTION = • EXAMPLE : What volume of 16 M sulfuric acid must be used to prepare 1.5 L of a 0.10 M H2SO4 solution?