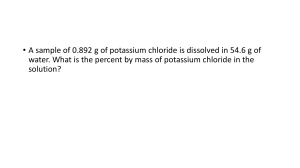STOICHIOMETRY OF REACTIONS IN SOLUTIONS LOUIS ANDREW R. TAYAG LEARNING COMPETENCY USE DIFFERENT WAYS OF EXPRESSING CONCENTRATION OF SOLUTIONS: PERCENT BY MASS, MOLE FRACTION, MOLARITY, MOLALITY, PERCENT BY VOLUME, PERCENT BY MASS, PPM. PERFORM STOICHIOMETRIC CALCULATIONS FOR REACTIONS IN SOLUTION. WHAT IS CONCENTRATION? WHAT IS CONCENTRATION? It is the amount of solute present in a solution. COMMON UNITS OF CONCENTRATION • Percent by mass • Percent by volume • Mole Fraction • Molarity • Molality PERCENT BY MASS Ratio of the mass of solute to the mass of the solution multiplied by 100% 𝑚𝑎𝑠𝑠 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑒 𝑚𝑎𝑠𝑠 𝑝𝑒𝑟𝑐𝑒𝑛𝑡 = 𝑥 100 𝑚𝑎𝑠𝑠 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑒 + 𝑚𝑎𝑠𝑠 𝑜𝑓 𝑠𝑜𝑙𝑣𝑒𝑛𝑡 𝑚𝑎𝑠𝑠 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑒 𝑚𝑎𝑠𝑠 𝑝𝑒𝑟𝑐𝑒𝑛𝑡 = 𝑥 100 𝑚𝑎𝑠𝑠 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑖𝑜𝑛 A sample of 0.678 g of sulfuric acid is dissolved in 65 g of water. What is the percent by mass of H2SO4 in the solution? A saline solution with a mass of 355 g has 36.5 g of salt dissolved in it. What is the mass percent concentration of the solution? ppm Ratio of the mass of solute to the mass of the solution multiplied by 106 𝑚𝑎𝑠𝑠 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑒 6 𝑝𝑝𝑚 = 𝑥 10 𝑚𝑎𝑠𝑠 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑖𝑜𝑛 Find the concentration of calcium ion (in ppm) in a 3.50 g pill that 2+. contains 40.5 mg of Ca Find the concentration of HCl (in ppm) in a 20.50 g solution that contains 4 g of HCl. PERCENT BY VOLUME Ratio of the volume of solute to the volume of the solution multiplied by 100% 𝑣𝑜𝑙𝑢𝑚𝑒 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑒 𝑣𝑜𝑙𝑢𝑚𝑒 𝑝𝑒𝑟𝑐𝑒𝑛𝑡 = 𝑥 100 𝑣𝑜𝑙𝑢𝑚𝑒 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑖𝑜𝑛 Calculate the volume percent of a solution that is prepared by mixing 519.2 mL of helium and 168.4 mL of molecular chlorine. What is the volume percent of a solution that has 5.0 mL of hydrochloric acid (HCl) diluted to 100 mL with deionized water? MOLARITY Number of moles of solute in 1 liter solution. 𝑎𝑚𝑜𝑢𝑛𝑡 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑒 (𝑚𝑜𝑙) 𝑀= 𝑣𝑜𝑙𝑢𝑚𝑒 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑖𝑜𝑛 (𝐿) Calculate the molarity of 5.80 g of LiNO3 in 505 mL of solution. Calculate the molarity of 0.444 mol of CoCl2 in 0.654 L of solution. MOLALITY Number of moles of solute in dissolved in 1kg of solvent. 𝑎𝑚𝑜𝑢𝑛𝑡 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑒 (𝑚𝑜𝑙) 𝑚= 𝑚𝑎𝑠𝑠 𝑜𝑓 𝑠𝑜𝑙𝑣𝑒𝑛𝑡 (𝑘𝑔) Calculate the molality of a sulfuric acid solution containing 24.4 g of sulfuric acid in 198 g of water. The molar mass of sulfuric acid is 98.09 g/mol. Calculate the molality of a solution prepared from 29.22 g of NaCl in 2.00 kg of water. MOLE FRACTION Number of moles of a component divided by the total number of moles of a solution. 𝑎𝑚𝑜𝑢𝑛𝑡 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑒 (𝑚𝑜𝑙) 𝑋= 𝑎𝑚𝑜𝑢𝑛𝑡 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑖𝑜𝑛 (𝑚𝑜𝑙) A sample of rubbing alcohol contains 142 g of isopropyl alcohol (C3H7OH) and 58.0 g of water. What are the mole fractions of alcohol and water? Determine the mole fraction of CH3OH and H2O in a solution prepared by dissolving 5.5 g of alcohol in 40 g of H2O.