Lewis Dot Structures
advertisement
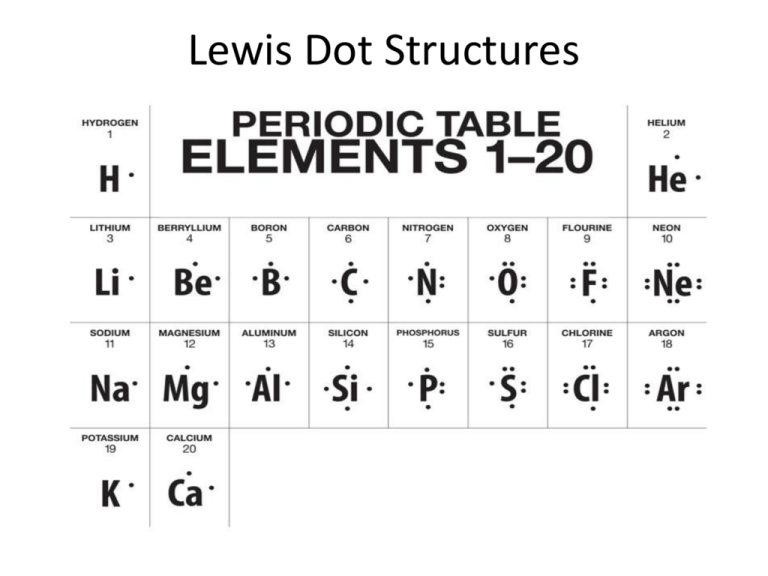
Lewis Dot Structures What are they? • Lewis dot structures of individual atoms depict the number of valence electrons an atom has. – For example: • They are used to represent the bonds between atoms and the remaining lone pairs of valence electrons. – For example: (water) (carbon dioxide) STEP 1 Determine the number of valence electrons (outer shell electrons) each atom has. a) Water (H2O) b) Carbon dioxide (CO2) STEP 2 Find the TOTAL number of valence electrons in the compound. a) Water (H2O) 6 + b) Carbon dioxide (CO2) 4 + 6 + 6 1 + 1 = 8 total valence electrons = 16 total valence electrons STEP 3 Arrange the atoms. If there is more than one atom type in the molecule, put the least electronegative atom in the center. a. Recall that electronegativity decreases as atom moves further away from fluorine on the periodic chart. b. EXCEPTION: Hydrogen is always placed on the outside of the compound STEP 3 (cont’d) Arrange the atoms: a) Water (H2O) b) Carbon dioxide (CO2) STEP 4 Arrange the electrons so that each atom contributes one electron to a single bond between each atom. a) Water (H2O) b) Carbon dioxide (CO2) H H STEP 5 Count the electrons around each atom – are the octets complete (for hydrogen – is the duet complete)? If so, your Lewis dot structure is complete. H a) Water (H2O) 8 electrons around oxygen = complete octet! H 2 electrons around hydrogen = complete duet! STEP 5 (cont’d) b) Carbon dioxide (CO2) 7 electrons around oxygen = not complete octet 6 electrons around carbon = not complete octet STEP 6 If the octets are incomplete, and more electrons remain to be shared, move one electron per bond per atom to make another bond. b) Carbon dioxide (CO2) STEP 7 Any electrons that are being shared by two atoms represent covalent bonds. Redraw the electron dots as lines for those bonds. a) Water (H2O) b) Carbon dioxide (CO2) Try some on your own On your worksheet try problems 1 and 2. Follow the steps 1-7 to draw the Lewis dot structures for these molecular compounds: 1. PBr3 2. N2H2 Answers 1. PBr3 2. N2H2 Problem #3 3. CH3OH – Remember to put the least electronegative atom in the center (C) and hydrogens belong on the outside of the structure (always!). Problem #3 3. CH3OH Problem #4 When we need to represent a polyatomic ion, the charge indicates that there is an extra electron involved. 4. NO2-1 – – – – Nitrogen = 5 valence electrons Oxygen = 6 valence electrons Oxygen = 6 valence electrons -1 charge = 1 valence electron TOTAL = 18 valence electrons Problem #4 4. NO2-1 – Since we have an extra electron, brackets are used around the entire structure and the -1 charge is indicated on the outside Problem #5 Try building dicarbon dihydride (C2H2) using the Molecular Modeling Kit. 5. C2H2 – Read the instructions page in the kit to help identify which color balls represent which element. Problem #5 5. C2H2 – It should look something like this: Complete the packet Use the Modeling Kits to aid you in drawing the Lewis structures for the rest of the molecules in your packet.



