Week 4 2-3 Carbon Compounds Pgs 44
advertisement

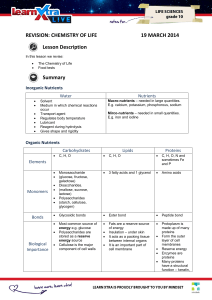
Week 4 2-3 Carbon Compounds Pgs 44-48 1. Name four groups of organic compounds found in living things. Carbohydrates, lipids, nucleic acids and proteins Questions 2. Describe at least one function of each group of organic compounds. A typical response might mention that living things use carbohydrates as their main source of energy, fats can be used to store energy, and nucleic acids transmit hereditary information and proteins form tissues. 3. What properties of carbon explain carbon’s ability to form many different macromolecules? Each carbon atom can form four covalent bonds, and carbon atoms can bond with other carbon atoms. 4. Explain why proteins are considered polymers but lipids are not. Proteins are made up of amino acid monomers joined in long chains. Although fatty acid chains may be mistaken for monomers, only three fatty acids can attach to a glycerol molecule. 5. Compare the structures and functions of the biomolecules lipids and starches. Lipids are made mostly from carbon and hydrogen atoms; starch is a carbohydrate made up of carbon, hydrogen and oxygen atoms. Both can be used to store energy. Science as a Way of Knowing Use what you learned about levels of organization in Section 1-3 to discuss the levels of organization in macromolecules. Begin your discussion with the smallest structure. 2-4 1. Chemical Reactions/Enzymes Pgs 49-53 Questions What happens to chemical bonds during chemical reactions? Bonds are broken in reactants and new bonds are formed in products. 2. Describe the role of energy in chemical reactions. Some chemical reactions release energy, and other chemical reactions absorb energy. Energy changes determine how easily a chemical reaction will occur. 3. What are enzymes, and how are they important to living things? Enzymes are biological catalysts. Living cells use enzymes to speed up virtually every important chemical reaction that takes place in cells. 4. Describe how enzymes work, including the role of the enzyme-substrate complex. Substrates, the reactants of an enzyme-catalyzed reaction, attach to the enzyme at an active site and form an enzyme-substrate complex. Once the complex is formed, the enzyme helps convert substrate into product. 5. A change in pH can change the shape of a protein. How might a change in pH affect the function of an enzyme such as hexokinase? Hint: Think about the analogy of the lock and key. A change in pH could change the shape of hexokinase. This change would diminish or possibly eliminate the ability of glucose and ATP to bind to the active site on the enzyme. Modeling Make a model that demonstrates how an active site and a substrate are like a lock and a key. Give a brief talk in which you refer to your model as you explain how enzymes work. 38-1 1. Food and Nutrition Pgs 971-977 Questions List the six nutrients needed by the body. Water, carbohydrates, fats, proteins, vitamins and minerals 2. What is the importance of water in the body? Some body tissues, such as blood, are mostly water, and water is needed for many vital body processes, including chemical reactions, elimination of wastes, and keeping the body cool through evaporation. 3. Why is fiber an important part of your diet? Fiber adds bulk to the material moving through the digestive system, helping it to process food more effectively. 4. How are vitamins and minerals similar? How are they different? Both vitamins and minerals are nutrients that are needed in small amounts for good health, but vitamins are organic molecules, whereas minerals are inorganic. 5. Which vitamins and minerals promote healthy bones? Vitamins C and D, calcium, phosphorus, and fluorine Designing a Brochure Design and create a brochure that explains how the body uses the six nutrients necessary for normal function. Use images from magazines or from the Internet to illustrate your brochure.