5.6 Chemical Equations
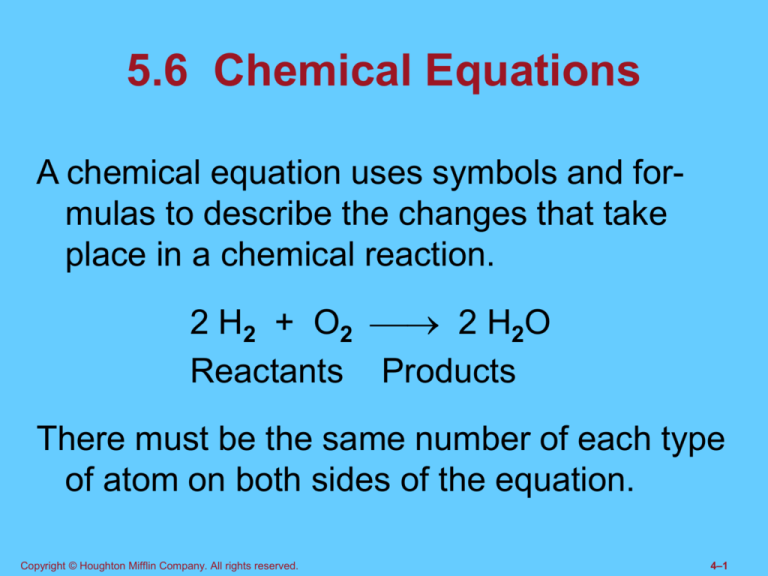
A chemical equation uses symbols and formulas to describe the changes that take
place in a chemical reaction.
2 H2 + O2 2 H2O
Reactants Products
There must be the same number of each type
of atom on both sides of the equation.
Copyright © Houghton Mifflin Company. All rights reserved.
4–1
5.6 Chemical Equations
A skeleton equation has the same type, but
not the same number, of each type of atom
on each side of the equation. It must be
balanced before it is useful in chemical
calculations.
Fe2O3 + CO Fe + CO2
Copyright © Houghton Mifflin Company. All rights reserved.
4–2
5.6 Chemical Equations
More equations to balance:
C3H8 + O2 CO2 + H2O
C4H10 + O2 CO2 + H2O
Al2O3 + HCl AlCl3 + H2O
II–1
Copyright © Houghton Mifflin Company. All rights reserved.
4–3
5.6 Chemical Equations
We can read equations as referring to
individual atoms and molecules, but
in the real world, we work with very
large groups of atoms and molecules.
Copyright © Houghton Mifflin Company. All rights reserved.
4–4
5.2 The Mole: A Counting Unit
for Chemists
We often use words that refer to a specific
number of items:
a pair of socks = 2 socks
a dozen eggs = 12 eggs
fourscore years = 4 x 20 years
It would be convenient to work with a specific
number of atoms in chemical calculations.
Copyright © Houghton Mifflin Company. All rights reserved.
4–5
5.2 The Mole
In 12.0 g of C-12, there are 6.022 x 1023 atoms.
6.022 x 1023 objects is one mole of objects
6.022 x 1023 is “Avogadro’s number”
A mole is a huge number, but we can use it like
we use dozens and scores.
Copyright © Houghton Mifflin Company. All rights reserved.
4–6
5.3 Molar Masses
6.022 x 1023 atoms of C-12 have a mass
of 12.0 grams.
The mass of a mole of atoms of all other
elements is defined relative to the mass
of 1 mole of C-12.
Atomic mass of Mg = 24.305 amu
Molar mass of Mg = 24.305 g/mol
Copyright © Houghton Mifflin Company. All rights reserved.
4–7
5.3 Molar Masses
We can obtain a mole of an element by weighing it. If we need more or less than a mole, we
can calculate the desired amount. We can
also determine how many atoms are present.
Atoms
Moles
Molecules
Avogadro’s #
Molar mass
6.022 x 1023
mole
grams
mole
Copyright © Houghton Mifflin Company. All rights reserved.
4–8
5.3 Molar Masses
Use the molar mass of magnesium and
Avogadro’s number as needed to make
these calculations:
How many moles of Mg in 12.8 g of Mg?
How many grams of Mg in 2.50 mol of Mg?
How many atoms in 2.50 mol of Mg?
How many atoms in 42.8 g of Mg?
Copyright © Houghton Mifflin Company. All rights reserved.
4–9
5.4 Moles and Chemical Formulas
1 Molecule of H2O 2 H atoms
1 O atom
1 Mole of H2O
2 moles H atoms
1 mole O atom
We work in moles, rather than
molecules, because we can’t
see individual molecules!
Copyright © Houghton Mifflin Company. All rights reserved.
4–10
5.4 Moles and Chemical Formulas
In a reaction equation:
2 H2 + O2 2 H2O
2 H2 molecules and 1 O2 molecule
produce 2 H2O molecules.
2 moles of H2 molecules and 1 mole
of O2 molecule produce 2 moles
of H2O molecules.
Copyright © Houghton Mifflin Company. All rights reserved.
4–11
5.4 Moles and Chemical Formulas
What is the mass of 1.00 mole of H2O?
How many H2O molecules are present in
one mole of water?
Copyright © Houghton Mifflin Company. All rights reserved.
4–12
5.5 Calculations with Molar Mass
A can of soda contains 340 g of water.
How many moles of water is this?
How many grams of hydrogen are
present in this amount of water?
How many hydrogen atoms are present in this amount of water?
Copyright © Houghton Mifflin Company. All rights reserved.
4–13
5.7 Chemical Equations and Moles
N2 + 3 H2 2 NH3
3 moles of H2 produce 2 moles of NH3
3 mol H2
2 mol NH3
Copyright © Houghton Mifflin Company. All rights reserved.
2 mol NH3
3 mol H2
4–14
5.8 Mass Calculations for
Chemical Equations
A person eats a candy bar that contains
14.2 g of sucrose (C12H22O11). How
much water will be produced by the
sucrose? It is metabolized according
to the equation shown below:
C12H22O11 + 12 O2 12 CO2 + 11 H2O
II-2
Copyright © Houghton Mifflin Company. All rights reserved.
4–15
5.8 Mass Calculations
How much Fe2O3 must I use if I want
500 g of Fe metal?
Fe2O3 + 3 CO 2 Fe + 3 CO2
NaI and CH4O react accordingly:
NaI + CH4O CH3I + NaOH
If one starts with 750 g each of NaI
and CH4O, how much CH3I will be
produced?
Copyright © Houghton Mifflin Company. All rights reserved.
4–16




