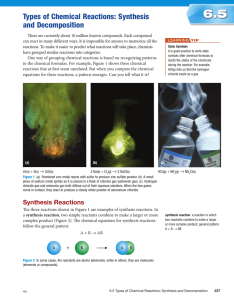
Chemical Reactions
advertisement
Chemical Reactions 4.2 Synthesis and Decomposition Reactions Last Class • Reviewed chemical reactions and chemical equations • Reviewed how to balance chemical equations Learning Goals • We are learning to… – Identify and predict the products of synthesis and decomposition reactions Chemical Reactions • Most chemical reactions can be grouped into 4 categories 1. 2. 3. 4. Synthesis Decomposition Single displacement Double displacement Synthesis Reactions Two reactants combine to make a larger/more complex product. General Formula: A + B AB Example: Synthesis of sodium chloride Na(s) + Cl(g) NaCl(s) Example Write the balanced chemical equation for the reaction of magnesium with oxygen 1. 2. 3. 4. 5. Identify the reactants Identify the type of rxn Predict products Skeleton equation Balance Synthesis Reactions (non-metals) Reactions involving hydrogen • Follow a similar pattern as those involving metals. Although covalent, you can use charges to predict products • Ex: H2(g) + Cl2(g) 2HCl (g) – We can apply the ionic changes of H (+1) and Cl (-1) Example Write the balanced chemical equation for the reaction of hydrogen with oxygen 1. 2. 3. 4. 5. Identify the reactants Identify the type of rxn Predict products Skeleton equation Balance Synthesis Reactions (non-metals) Reactions not involving hydrogen • Products are difficult to predict • Ex: Depending on reaction conditions C(s) + O2(g) CO2(g) or 2C(s) + O2(g) 2CO(g) Synthesis Reactions (non-metals) Reactions involving compounds • Products may be difficult to predict • Ex: water and carbon dioxide react to form carbonic acid H2O(l) + CO2(g) H2CO3(aq) Example Water reacts with sulfur trioxide in a synthesis reaction to form sulfuric acid – Write the balanced chemical equation, include states. Decomposition Reactions • A reaction in which a larger compound breaks down to form two (or more) simpler products • General Formula: AB A + B • Ex: The decomposition of potassium chloride 2KCl(s) 2K(s) + Cl2(g) Decomposition Reactions • Decomposition reactions involving polyatomic ions or molecular compounds can be difficult to predict – do not break apart like binary ionic compounds • Ex: potassium chlorate decomposes to produce potassium chloride and oxygen KClO3(s) KCl(s) + O2(g) Example • calcium carbonate decomposes to produce calcium oxide and carbon dioxide • Write the balanced chemical equation, include states. Homework page 161 # 1-4, 6, 7, 9