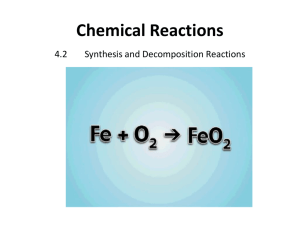
Synthesis & Decomposition Reactions: Chemistry Textbook Excerpt
advertisement
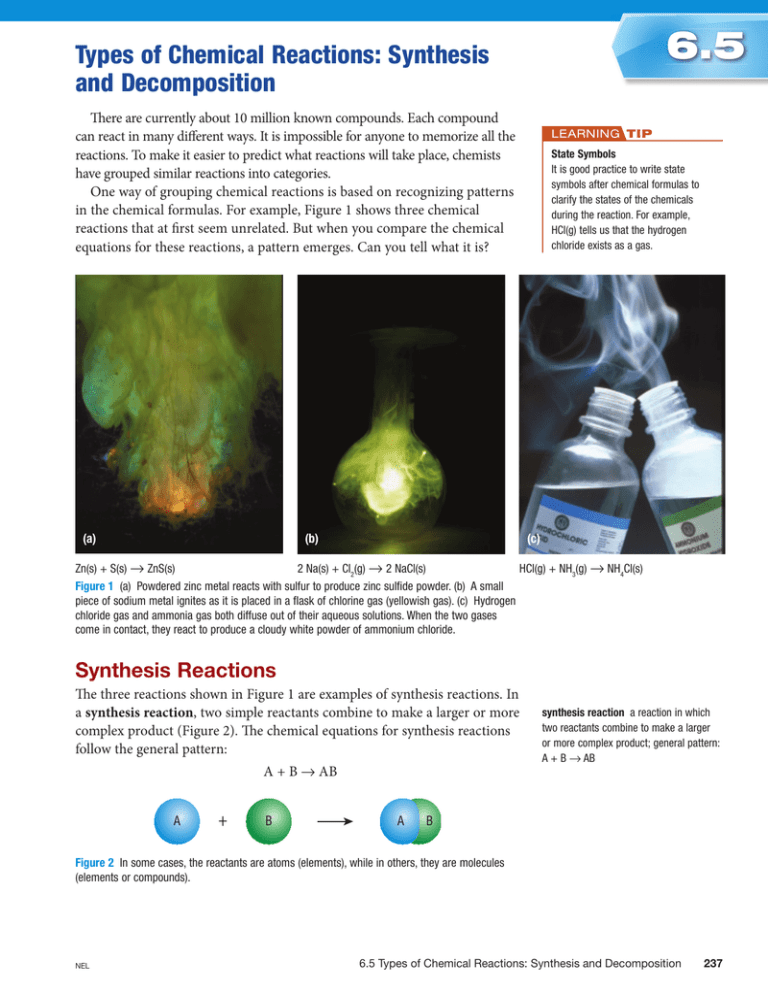
6.5 Types of Chemical Reactions: Synthesis and Decomposition There are currently about 10 million known compounds. Each compound can react in many different ways. It is impossible for anyone to memorize all the reactions. To make it easier to predict what reactions will take place, chemists have grouped similar reactions into categories. One way of grouping chemical reactions is based on recognizing patterns in the chemical formulas. For example, Figure 1 shows three chemical reactions that at first seem unrelated. But when you compare the chemical equations for these reactions, a pattern emerges. Can you tell what it is? (a) (b) LeaRning Tip State Symbols It is good practice to write state symbols after chemical formulas to clarify the states of the chemicals during the reaction. For example, HCl(g) tells us that the hydrogen chloride exists as a gas. (c) Zn(s) + S(s) → ZnS(s) 2 Na(s) + Cl2(g) → 2 NaCl(s) HCl(g) + NH3(g) → NH4Cl(s) Figure 1 (a) Powdered zinc metal reacts with sulfur to produce zinc sulfide powder. (b) A small piece of sodium metal ignites as it is placed in a flask of chlorine gas (yellowish gas). (c) Hydrogen chloride gas and ammonia gas both diffuse out of their aqueous solutions. When the two gases come in contact, they react to produce a cloudy white powder of ammonium chloride. synthesis Reactions The three reactions shown in Figure 1 are examples of synthesis reactions. In a synthesis reaction, two simple reactants combine to make a larger or more complex product (Figure 2). The chemical equations for synthesis reactions follow the general pattern: A + B → AB A + B A synthesis reaction a reaction in which two reactants combine to make a larger or more complex product; general pattern: A + B → AB B Figure 2 In some cases, the reactants are atoms (elements), while in others, they are molecules (elements or compounds). NEL 6.5 Types of Chemical Reactions: Synthesis and Decomposition 237 Table 1 shows how the three synthesis reactions in Figure 1, on the previous page, follow the general pattern. Table 1 Examples of Synthesis Reactions Synthesis reaction Equation zinc sulfide (Figure 1(a)) zinc + sulfur → zinc sulfide Zn(s) + S(s) → ZnS(s) sodium chloride (Figure 1(b)) sodium + chlorine → sodium chloride 2 NaCl(s) 2 Na(s) + Cl2(g) → ammonium chloride (Figure 1(c)) ammonia + hydrogen chloride → ammonium chloride HCl(g) → NH4Cl(s) NH3(g) + General pattern A + B → AB Decomposition Reactions decomposition reaction a reaction in which a large or more complex molecule breaks down to form two (or more) simpler products; general pattern: AB → A + B We can think of decomposition reactions as being the opposite of synthesis reactions. During a decomposition reaction, large compounds are broken down into smaller compounds or elements (Figure 3). The general pattern for decomposition reactions is AB → A + B A A B + B Figure 3 In a decomposition reaction, a complex molecule breaks down, or decomposes, into simpler products. The products can be elements or compounds. LeaRning Tip Just the Opposite Decomposition reactions (AB → A + B) are the reverse of synthesis reactions (A + B → AB). Decomposition reactions usually absorb energy (such as thermal or electrical energy) from an external source. This energy is then used to convert reactants into products. For example, water can be decomposed into its elements using electricity as its energy source. C06-F14-UBOS10SB.ai Table 2 shows how two decomposition reactions follow the general pattern. Table 2 Examples of Decomposition Reactions Decomposition reaction Equation water energy + water → hydrogen + oxygen energy + 2 H2O(l) → 2 H2(g) + O2(g) sodium azide energy + sodium azide → sodium + nitrogen 2 NaN3(s) → 2 Na(s) + 3 N2(g) Ontario Science 10 SB General pattern AB → A + B 0-17-635528-6 C06-F14-UBOS10SB FN Many nitrogen compounds undergo important decomposition reactions. CrowleArt Group CO For example, Deborah Crowle the use of airbags in cars is a lifesaving application of a Figure 4 Airbags are designed to slow decomposition reaction (Figure 4). Airbags contain a nitrogen compound Pass your forward motion during a collision. 2nd pass An airbag inflatesApproved in about 1/20th of a second and staysNot fully Approved inflated for only about 1/10th of a second. 238 called sodium azide, NaN3(s).Duringacollision,asuddenflowofelectricity is automatically sent to the airbag. This electrical energy triggers the rapid decomposition of sodium azide to produce nitrogen gas and sodium metal. Chapter 6 • Chemicals and Their Reactions NEL RESEARCH THIS PROPOSaL TO Ban FeRTiLiZeRS SKILLS: Researching, Analyzing the Issue, Defending a Decision, Communicating SKILLS HANDBOOK 4.A., 4.C. Ammonium nitrate is one of the cheapest and most widely used fertilizers. However, it can also be used to make explosives (Figure 5): ammonium nitrate → water + nitrogen + oxygen + energy 2 NH4NO3(s) → 4 H2O(g) + 2 N2(g) + O2(g) + energy Because of this, some politicians have proposed that the sale of ammonium nitrate should be restricted or banned. 1. Research the facts regarding the use of ammonium nitrate as a fertilizer and in the manufacture of explosives. 2. Research the arguments for and against the proposed ban. 3. Organize your findings in a “pros” and “cons” chart. T/I C A. Do you think the proposed ban is fair? Defend your decision in a letter to a local politician. C in Figure 5 A few well-placed explosive charges can demolish old, vacant buildings quickly and safely. Many explosives release their destructive energy through decomposition reactions. SUMMARY •Chemicalreactionsaregroupedintocategories. Within each category, the reactions follow the same pattern. •Inasynthesisreaction,twosimplereactants combine to make a larger or more complex product and follow the general pattern A + B → AB. CHECK •Inadecompositionreaction,acomplexreactant breaks down to make two or more simpler product and follow the general pattern AB → A + B. YOUR LeaRning 1. Classify each of the following as either a synthesis or a decomposition reaction: K/U 4. Write a balanced chemical equation for each of the following reactions. Include state symbols. Classify each one as either synthesis or decomposition. K/U T/I (a) zinc chloride → zinc + chlorine (a) Hydrogen gas reacts explosively with chlorine gas to form hydrogen chloride gas. (b) potassium + iodine → potassium iodide (c) potassium oxide + water → potassium hydroxide (d) calcium carbonate → calcium oxide + carbon dioxide 2. Write a balanced chemical equation for each of the word equations given in question 1. K/U T/I 3. Copper metal was first made over 3 000 years ago by heating a mineral containing copper(II) oxide. The other product of this reaction is oxygen. K/U T /I (b) A solution of hydrogen peroxide, H2O2, breaks down into water and oxygen. (c) Solid potassium chlorate breaks down into solid potassium chloride and oxygen when heated. (d) Ammonia gas, NH3, can be made by combining hydrogen gas and nitrogen gas. (e) Aluminum metal reacts with oxygen from the air to form a hard coating of aluminum oxide. This coating prevents aluminum objects from corroding. (a) Write a word equation for this reaction. (b) Is this a synthesis reaction or a decomposition reaction? Explain. (c) Write a balanced chemical equation for the reaction. NEL 6.5 Types of Chemical Reactions: Synthesis and Decomposition 239