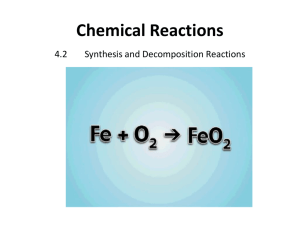
Synthesis and Decomposition Reactions
advertisement

NAME ________________________________________ PERIOD ________ DATE _______ Synthesis and Decomposition Reactions Complete the following study guide as you watch the Synthesis and Decomposition Reactions Video OR read pages 189-190 in your chemistry textbook. There are 5 types of chemical reactions that we will talk about: _____________________ ________________________________________________________________________ **You need to be able to identify the type of reaction, predict the product(s), and balance. Remember your 7 diatomic elements, and how to write formulas for compounds! Synthesis reactions are chemical reactions in which two or more substances (generally elements) react to form a new product. The general form of a synthesis reaction is written as: A + B AB Examples: Predict the products. Write and balance the following synthesis reaction equations. •Sodium metal reacts with chlorine gas to form… •Solid magnesium reacts with fluorine gas to form… •Aluminum metal reacts with liquid bromine to form... Write the reactants of the following synthesis reactions (the product is given): 1. Synthesis of CaO: 2. Synthesis of nitrogen trioxide: 3. Synthesis of chromium (III) oxide: 4. Synthesis of NaI: NAME ________________________________________ PERIOD ________ DATE _______ Decomposition reactions are chemical reactions in which a reactant (a compound) breaks down into two or more products. The general form of a decomposition reaction is written as: AB A + B Examples: Predict the products. Then, write and balance the following decomposition reaction equations: •Solid Lead (II) oxide decomposes •Aluminum nitride decomposes Guidelines for Decomposition Reactions (The reverse is also true for synthesis reactions): • Binary compounds breakdown into their elements • • Carbonates (CO32-) decompose to carbon dioxide and a metal oxide • • Example: CaCO3 CO2 + CaO Chlorates (ClO3-) decompose to oxygen gas and a metal chloride • • Example: 2NaCl (s) 2Na (s) + Cl2 (g) Example: 2 Al(ClO3)3 2 AlCl3 + 9 O2 Compounds with a hydroxide ion break down to the oxide of the metal and water • Example: Ca(OH)2 (s) CaO (s) + H2O (l) Write the products in the following decomposition reactions (the reactant is given): 1. Decomposition of Ag2O: 2. Decomposition of potassium chloride: 3. Decomposition of KClO3: