Atoms and Elements Review Answer Key
advertisement
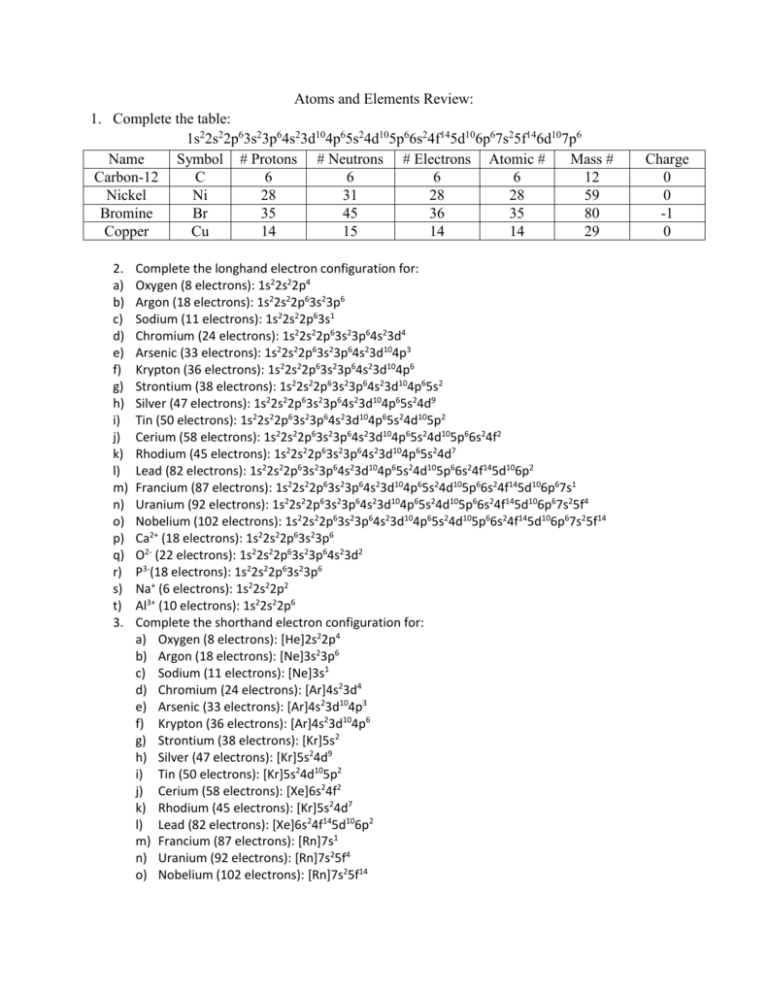
Atoms and Elements Review: 1. Complete the table: 1s22s22p63s23p64s23d104p65s24d105p66s24f145d106p67s25f146d107p6 Name Symbol # Protons # Neutrons # Electrons Atomic # Mass # Carbon-12 C 6 6 6 6 12 Nickel Ni 28 31 28 28 59 Bromine Br 35 45 36 35 80 Copper Cu 14 15 14 14 29 2. a) b) c) d) e) f) g) h) i) j) k) l) m) n) o) p) q) r) s) t) 3. Complete the longhand electron configuration for: Oxygen (8 electrons): 1s22s22p4 Argon (18 electrons): 1s22s22p63s23p6 Sodium (11 electrons): 1s22s22p63s1 Chromium (24 electrons): 1s22s22p63s23p64s23d4 Arsenic (33 electrons): 1s22s22p63s23p64s23d104p3 Krypton (36 electrons): 1s22s22p63s23p64s23d104p6 Strontium (38 electrons): 1s22s22p63s23p64s23d104p65s2 Silver (47 electrons): 1s22s22p63s23p64s23d104p65s24d9 Tin (50 electrons): 1s22s22p63s23p64s23d104p65s24d105p2 Cerium (58 electrons): 1s22s22p63s23p64s23d104p65s24d105p66s24f2 Rhodium (45 electrons): 1s22s22p63s23p64s23d104p65s24d7 Lead (82 electrons): 1s22s22p63s23p64s23d104p65s24d105p66s24f145d106p2 Francium (87 electrons): 1s22s22p63s23p64s23d104p65s24d105p66s24f145d106p67s1 Uranium (92 electrons): 1s22s22p63s23p64s23d104p65s24d105p66s24f145d106p67s25f4 Nobelium (102 electrons): 1s22s22p63s23p64s23d104p65s24d105p66s24f145d106p67s25f14 Ca2+ (18 electrons): 1s22s22p63s23p6 O2- (22 electrons): 1s22s22p63s23p64s23d2 P3-(18 electrons): 1s22s22p63s23p6 Na+ (6 electrons): 1s22s22p2 Al3+ (10 electrons): 1s22s22p6 Complete the shorthand electron configuration for: a) Oxygen (8 electrons): [He]2s22p4 b) Argon (18 electrons): [Ne]3s23p6 c) Sodium (11 electrons): [Ne]3s1 d) Chromium (24 electrons): [Ar]4s23d4 e) Arsenic (33 electrons): [Ar]4s23d104p3 f) Krypton (36 electrons): [Ar]4s23d104p6 g) Strontium (38 electrons): [Kr]5s2 h) Silver (47 electrons): [Kr]5s24d9 i) Tin (50 electrons): [Kr]5s24d105p2 j) Cerium (58 electrons): [Xe]6s24f2 k) Rhodium (45 electrons): [Kr]5s24d7 l) Lead (82 electrons): [Xe]6s24f145d106p2 m) Francium (87 electrons): [Rn]7s1 n) Uranium (92 electrons): [Rn]7s25f4 o) Nobelium (102 electrons): [Rn]7s25f14 Charge 0 0 -1 0 4. Draw Aufbau diagrams for: a)Hydrogen b) Helium c) Lithium d) Carbon e) Nitrogen f) Oxygen g) Chromium h) Magnesium i) Iron j) Cobalt k) Ca2+ l) O2- m) P-3 n) Na+ o) Al3+ 5. What are isotopes? Give an example. Isotopes are atoms of the same element (THE SAME ATOMIC NUMBER AND THE SAME NUMBER OF PROTONS), but with different number of neutrons (and as a result, a different mass number). For example, carbon – 12, carbon – 13, and carbon – 14. 6. If an element typically comes in three varieties with abundances of: Mass (g/mol) Abundance 45 18.4% 46 32.9% 47 48.7% What would be the mass that would be recorded on the periodic table? Write the symbolic representations for the isotopes of the element X. Mass = 46.303 g/mol 45 46 47 nX nX nX (n= protons in this case of unknown atomic number) 7. Give three IONS that have the electron configuration of 1s22s22p63s23p6. S2-, Ca2+, Cl-, P3-, K+ 8. Identify all the trends: 9. Explain why atomic radius decreases moving across a period and increases moving down a row. There is a decrease in atomic radius moving from left to right across the periodic table because there is an increasing nuclear charge due to the increase in PROTONS. This means that there is a greater pull on the outer most electrons. Moving down a group there is an increase in atomic radii. As the energy level increases and an electron orbital is added to the atom outer electrons move away from the nucleus creating a larger atom. 10.An element forms a negative ion when ionized. On what side of the periodic table is the element located? Explain. The element is located on the right side of the periodic table. Elements on the right side of the periodic table (ex. Group 15 – group 17) become stable when they gain a full octet of electrons. Therefore elements on the right side of the periodic table are more likely to gain an electron rather than lose an electron. Elements on this side of the periodic table are closer to becoming stable when they gain electrons, if they lose an electron they become less stable! 11. What is ionization energy? Ionization energy is defined as the energy required to remove an electron from a gaseous atom. Think of it as how strongly an atom’s nucleus holds onto its valence electrons. A strong hold means high ionizations energy and low ionization energy indicates an atom loses its outer electron easily. 12. Why does lithium have a low ionization energy. Li: 520KJ/mol (1st IE), 7300KJ/mol (2nd IE) Li is willing to lose one electron in order to become stable like He. We can see that as an atom loses an electron it requires more energy to move the next electron. As atoms and ions become stable it is almost impossible to strip an electron from the atom or ion because it requires high ionization energy! 13. What is electron affinity? Electron affinity is the change in energy when an electron is added to a gaseous atom or ion to from a negatively charged ion. Electron affinity increases across a period because more energy is released. The attraction between the incoming electron and the nucleus becomes stronger when we have a smaller atomic radius so more energy is released because it wants to add an electron. Electron affinity decreases moving down a group because electrons become farther away from the nucleus so there is less of an attraction. 14. Which element in each pair is more electronegative? Why? a) K, As b) N, Sb c) Sr, Be a) As is more electronegative than K because As wants to gain electrons and K wants to lose electrons in order for both of the atoms to be as stable as possible. b) N is more electronegative than Sb because Sb is larger, therefore it is easier for Sb to lose electron and for N to gain the electrons. c) Be is more electronegative than Sr because Sr is larger, therefore it is easier for Sr to lose electrons and easier for Be to gain electrons. 15. Who is Dmitri Mendeleev? Dmitri Mendeleev was a scientist who helped create the periodic table. He was different than the other scientists because he determined the missing elements on the periodic table by looking at the chemical and physical properties of the elements around it. 16. Where are the alkali metals in the periodic table? What trend do you notice when each metal reacts with water? The alkali metals are located in group 1 on the periodic table. The elements become more reactive with water as you go down the group because as the valence electrons move further from the nucleus they are more easily stripped from the atom. This causes them to react more violently in water. 17. Draw the Lewis structure and Bohr diagram for the following: Si, Mg+, S-. Si: 14 protons, 14 electrons, 14 neutrons Mg+: 12 protons, 11 electrons, 12 neutrons -1 Mg S-: 16 protons, 17 electrons, 16 neutrons 18. Use your timeline!


![The electronic configuration of phosphorus is [Ne] 3s2 3p3](http://s3.studylib.net/store/data/008974852_1-8381577ce936fbfa611892c1a5f109cd-300x300.png)



