Metallic Carbonates
advertisement

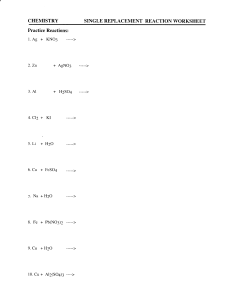
N2 + Li Na + Br2 Mg + I2 K+S Something breaks apart into individual pieces. Opposite of synthesis reactions. Couple breaks up Ex. 2NaCl 2Na + Cl2 You need to know four types Binary Metallic Carbonates Metallic Hydroxides Metallic Chlorates A compound breaks up into its elements AB A + B 2NaCl 2Na + Cl2 Exact opposite of synthesis! Decompose into the metal oxide and Carbon Dioxide MCO3 MO + CO2 PbCO3 PbO + CO2 Decompose into the metal oxide and water MOH MO + H2O Pb(OH)2 PbO + H2O Decompose into the metal chloride and oxygen gas MClO3 MCl + O2 Pb(ClO3)2 PbCl2 + 3O2 Determine the type of compound before trying to predict the products!! Use the “Guidelines for Predicting the Products of selected Types of Chemical Reaction” reference sheet Cu(OH)2 Li + O2 CaCl2 Mg + Cl2 Fe(ClO3)3 Na + Br2 NiCO3 K+S Unit 6, Day 4 Kimrey 31 October 2012 A Carbon containing compound reacts with oxygen. Carbon dioxide and water are always your products! So, predicting products is pretty simple. C6H12O6 + O2 H2O + CO2 Products will always be CO2 and H2O Look for a carbon containing compound and O2 as a reactants. The only work you have to do is balance the equation correctly. CH4 + O2 CH4 + 2O2 CO2 + 2H2O Cs + F2 2Cs + F2 2CsF Ba + Cl2 Ba + Cl2 BaCl2 C3H8 + O2 C3H8 + 5O2 3CO2 + 4H2O What is a single replacement reaction? One “kicks” out the less reactive element. Ex. 2Na (s) +2HCl(aq) 2NaCl(aq) + H2(g) Metals range in reactivity Some are very stable as elements ▪ These are less reactive ▪ Example: Gold, Au Some are very reactive ▪ These are mostly found in compounds (not by themselves) ▪ Example: Sodium, Na The most active metals are at the top of the series An elemental metal can replace any metallic ion that is BELOW it on the series If an element is more reactive, it will replace the less reactive ion in a compound. You will always be given the activity series as a reference table How to use the activity series. Find the element in the compound on the table. 2. Find the Solo element 3. If the solo element is above the element in the compound then the reaction will take place. 1. 2Al(s) + 3ZnCl2(aq) 3Zn(s) + 2AlCl3(aq) To replace the Zinc, Aluminum must be higher on the series Cu(s) + 2NaCl(aq) NO REACTION Can copper replace sodium in the compound? Above the activity series for metals, there is an activity series for Halogens. If your solo element is a halogen, it will replace the bonded halogen as long as it is above it on the activity series. Remember, every halogen on the series is a diatomic molecule, so when it’s by itself, there will be two of them (F2, Br2, …) • Cr(s) + Pb(NO3)2(aq) Cr(NO3)2(aq) + Pb(s) • Pt(s) + CaCl2(aq) NO REACTION • Ca(s) + FeO (aq) CaO (aq) + Fe(s)