wk2a
advertisement

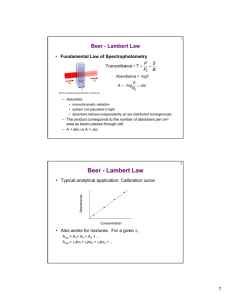
Absorption spectroscopy Summary: (Last lecture) • • • • • definition electromagnetic spectroscopy matter absorption spectroscopy fundamental terms (transmittance, absorbance absorptivity, molar absorptivity) Absorption spectroscopy Molar absorptivity (e) e A = ebc = the molar absorptivity, L mol-1 cm-1 (the characteristic of a substance that tells how much light is absorbed at particular wavelength) b = the pathlength of cell, cm C = the concentration of absorbing species, M Absorption spectroscopy Quantitative aspects of absorption measurements Beer’s Law A = ebc (The heart of spectrophotometry) *Application of Beer’s Law to mixture Absorption spectroscopy A solution containing more than one kind of absorbing substances: Atotal = A1 + A2 + … + An = e1bc1 + e2bc2 + … + enbcn Conditions: no interaction among the various species Absorption spectroscopy Limitations to the Applicability of Beer’s Law A a c • monochromatic radiation • dilute solutions ( 0.01 M) only Why? Absorption spectroscopy Limitations to the Applicability of Beer’s Law At high concentration (> 0.01M): • in concentrated solution, solutes molecules influence one another as a result of their proximity. When solute molecules get close to one another, their properties (including molar absorptivity) change somewhat. Absorption spectroscopy Limitations to the Applicability of Beer’s Law At high concentration (> 0.01M): • The solute becomes the solvent. Properties of a molecule are not exactly the same in different solvent. Absorption spectroscopy Deviations of Beer’s Law DEVIATIONS 1. Chemical Deviations 2. Instrumental Deviations • Polychromatic Radiation • Stray Light Deviations of Beer’s Law Chemical deviations • arise when an analyte dissociates, associates, or reacts with a solvent to produce having a different absorption spectrum from the analyte. Ex: acid/base indicators HIn colour 1 = H+ + Incolour 2 Ex: The molar absorptivity of the weak acid HIn (Ka=1.42 x 10-5) and its conjugate base In- at 430 and 570 nm were determined by measurements of strongly acidic and strongly basic solutions of the indicator (where essentially all of the indicator was in HIn and In- forms respectively). The results were e430 e570 HIn 6.30 x 102 7.12 x 103 In 2.06 x 104 9.61 x 102 Derive absorbance data for unbuffered solutions having total indicator concentrations ranging from 2 x 10-5 to 16 x 10-5 M Soln. Calculate the [HIn] and [In-] in a solution in which the [indicator] is 2.00 x 10-5 M Here HIn = H+ + In- [ H ][ In ] Ka 1 . 42 10 [ HIn ] 5 (1) From the equation for the dissociation process; [ H ] [ In ] [ In ] [ HIn ] 2 . 00 x 10 5 Substitution of these relationships into (1) for Ka: [ In ] 1 . 42 10 5 ( 2 . 00 10 ) [ In ] 2 5 [ In ] 1 . 12 10 5 [ HIn ] 2 . 00 10 5 0 . 88 10 5 1 . 12 10 5 From Beer’s Law: A e In b [ In ] e HIn b [ HIn ] A430 2 . 06 10 1 . 00 1 . 12 10 5 6 . 30 10 2 1 . 00 0 . 88 10 5 4 0 . 236 At 570 nm: A570 9 . 61 10 2 1 . 00 1 . 12 10 5 7 . 12 10 1 . 00 0 . 88 10 5 3 0 . 073 Note: The direction of curvature is opposite at the two wavelengths. Instrumental deviations • polychromatic radiation Consider a beam consisting of just two wavelengths and at , P0 A log e bc P P0 e bc 10 P e bc P P0 10 at , P P010 e bc When an absorbance measurement is made with radiation composed of both wavelengths, the measurement A, Am: ( P0 P0) Am log ( P P ) ( P0 P0) Am log e bc ( P0 10 P010 e bc ) Am log( P0 P0) log( P010 e bc P010 e bc ) when e e Am e bc In experiment, deviations from Beer’s Law resulting from the use of a polychromatic radiation is not appreciable. Instrumental deviations • stray light Causes: scattering and reflections from various internal surface Characteristic: • differs greatly in wavelength from that of the principal radiation • may not have passed through the sample Instrumental deviations • stray light P0 Ps A log P Ps Ps is the power of nonabsorbed stray radiation note At high concentration and at longer path lengths, stray radiation can also cause deviations from the linear relationship between ABS and path length. M.R. Share, Anal. Chem. 1984, 56, 339A Instrumental deviations: stray light Summary: The instrumental deviations result in absorbance that are smaller than theoretical. OR The instrumental deviations always lead to negative absorbance error. Analysis of Mixtures of Absorbing Substances : A e M bc M e N bc N : A eM bc M eN bc N • two components behave independently of one another. Example 1 The molar absorptivities of compounds X and Y were measured with pure samples of each. e (nm) 272 327 (M-1 cm-1) X Y 16440 3990 3870 6420 A mixture of compounds X and Y in a 1.000 cm cell has an absorbance of 0.957 at 272 and 0.559 at 327 nm. Find the concentrations of X and Y in the mixture. Example 2 The figure shows the spectra of 1.00x10-4 M MnO4-, 1.00x10-4 M Cr2O72-, and unknown mixture mixture of both. Absorbances at several wavelengths are given in the table. Find the concentration of each species in the mixture Wavelength (nm) 266 288 320 350 360 MnO4standard 0.042 0.082 0.168 0.125 0.056 Cr2O72standard 0.410 0.283 0.158 0.318 0.181 Mixture 0.766 0.571 0.422 0.672 0.366 Quiz 2: สารละลายของสารอินทรีย์ตัวหนึ่งเตรียมขึน้ จากสารละลาย 0.287 mg ในเอธานอล 10 mL พบว่ าหากใช้ เซลที่มี ความหนา 1.0 cm จะให้ ค่าการดูดกลืน 1.25 ที่ 305 nm จงคานวณ molar absorptivity กาหนดให้ นา้ หนักโมเลกุล ของสารเท่ ากับ 500 Summary: key terms Beer’s Law the relationship between a sample’s absorbance and the concentration of the absorbing species Stray Light any light reaching the detector that does not follow the optic path from the source to the detector Transmittance the ratio of the radiant power passing through a sample to that from the radiation’s source Absorbance The attenuation of photons as they pass through a sample (A) Absorbance spectrum a graph of a sample’s absorbance of electromagnetic radiation versus wavelength (frequency or wavenumber) photon a particle of light carrying an amount of energy equal to hv Next topic: Instruments for absorption measurements Instrument components: UV-VIS optical source hn1 signal processor selector hn2 detector sample Instrument components: UV-VIS Sources: A sources must: • generate a beam of radiation with sufficient power for easy detection and measurement • provide output power that is both stable and intense Types of spectroscopic sources: 1. continuous sources 2. lines sources Instrument components: UV-VIS continuous sources lines sources H2 and D2 lamp Tungsten filament lamps Xe arc lamp hollow cathode lamp Hg vapor lamp laser Instrument components: UV-VIS Tungsten filament lamp: Vis/near IR source • 320-2500 nm Instrument components: UV-VIS Quartz Tungsten Halogen (QTH) lamp • 200-3000 nm • high temperature (3500 K) Evaporation: W(s) W(g) + I2(g) Redeposition: WI2(g) + W(s) W(g) WI2(g) W(s) + I2(g) Instrument components: UV-VIS H2 and D2 lamp • provide continuous spectrum in the UV region (180-375 nm) by electrical excitation of deuterium or hydrogen at low pressure mechanism H2 + Eelectrical H2* H(KE1) + H(KE2) + hv KE 1 KE 2 hn E electrical BDE ‘bond dissociation energy’ Instrument components: UV-VIS sample containers Instrument components: UV-VIS sample containers Note: • a liquid sample is usually contained in a cell called a cuvet that has a flat • material • fused silica • glass only Vis • quartz Instrument components: UV-VIS wavelength selectors Types 1. Filters 1.1 interference filters 1.2 absorption filters 2. Monochromators Instrument components: UV-VIS Filters “a wavelength selector that uses either absorption, or constructive and destructive interference to control range of selected wavelengths” • the simplest method for isolating a narrow band of radiation Instrument components: UV-VIS Absorption filters • work by selectively absorbing radiation from a narrow region Interference filters • use constructive and destructive interference to isolate a narrow range of wavelengths Instrument components: UV-VIS Absorption filters • use coloured glass • provide effective bandwidths, range 30-250 nm the width of the band of radiation passing through a wavelength selector measured at half the band’s height Instrument components: UV-VIS Instrument components: UV-VIS Relationship between Absorption and Observed Colour wavelength region removed by absorption (nm) 400-450 450-480 480-490 490-500 500-560 560-580 580-600 600-650 650-750 colour observed violet blue green-blue blue-green green yellow-green yellow orange red complementary colour of the residual light, as seen by eye yellow-green yellow orange red purple violet blue green-blue blue-green