Names and Formulas for Ionic Compounds
advertisement

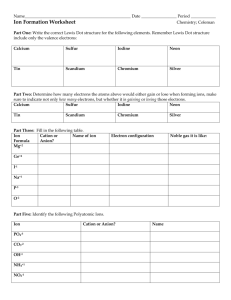
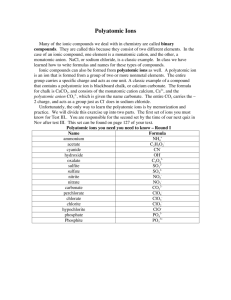
Names and Formulas for Ionic Compounds Types of ions • Monatomic ion – ion with one atom • Mg2+ magnesium ion • Cl- chloride ion • Polyatomic ion – ion with more than one atom • NH4+ • NO3- ammonium nitrate Ionic Compound Marriage Metals are like men Nonmetals are like woman Typically who changes their last name? Naming Ca”t”ions • Monatomic cation name stays the same – Sodium, Na goes to sodium ion, Na+ – Calcium, Ca goes to calcium ion, Ca 2+ • Polyatomic cation - look up in chart –NH4+ ammonium Naming Anions • The name changes in a monatomic anion • The ending for a monatomic anion is ______ide (like a bride changes her last name) – chlorine, Cl goes to chloride ion, Cl– Fluorine, F goes to fluoride ion, F- • Polyatomic anion look up in chart – OH- hydroxide – SO42- sulfate Question Time Given the neutral atom, give the charge and name of the monatomic ion that will form. (Look at periodic table group numbers for help.) 1. 2. 3. 4. 5. Na Cl Mg S P Na+ ClMg2+ S2P3- sodium cation chloride anion magnesium cation sulfide anion phosphide anion Name the following polyatomic ions. (use your chart!) 6.NH4+ ammonium cation hydroxide anion 7.OH− 8.SO32− sulfite anion 9.SO42− sulfate anion cyanide anion 10.CN− Naming ionic compounds • Name the cation first and then the anion second (+ then -) • Example: CsF – Monatomic cations use the element name • Cs+ = cesium – Monatomic anions – change the ending to ide • F - = fluoride cesium fluoride Will these form an Ionic Compound? If so what will the compound name be? 1. 2. 3. 4. 5. Mg Al Mg Na C Al S O N S No Yes two metals aluminum sulfide Yes magnesium oxide Yes No sodium nitride two non-metals Formula Unit • Formula Unit—the smallest whole number ratio of ions in a compound • NaCl versus Na2Cl2 • Correct: Sodium Chloride NaCl What will the formula be? In ionic compounds, we must balance charges to form a neutral compound To do that, we “crisscross the charges” Always reduce to get the formula unit Ca + N Ca2+ + N3Ca3 N2 Both have a total charge of 6 (3 Ca2+ = +6; 2 N3- = -6) Practice 1. 2. 3. 4. 5. Given the following elements, predict the formula. (hint first identify the ion charge) K, Br Ca, Cl Li, S Mg, N Ba, P Formula Unit with Polyatomic ions • Sometimes you will need to use more than one polyatomic ion to balance a charge. • If so, you need to put it in parenthesis (like a package) and use a subscript for the multiple number Ex: Mg2+ and NO3These charges do not balance so we must crisscross them Mg1(NO3)2 (we don’t need the 1) Mg (NO3)2 Transition Metal Ions • Metals form cations • Some transition metals only have one ionic charge – Silver (Ag+), Cadmium (Cd2+), Zinc (Zn2+) • Some transition metals can take on more than one charge. – Copper (Cu+, Cu2+) • Charge is determined from the number of electrons lost Naming Transition Metal Ions • If a transition metal has more than one charge, the charge is written as a Roman numeral in parenthesis • Some metals need a middle name • Fe (iron) has more than one charge: 2+ and 3+ – Fe2+ – Fe3+ iron(II) iron(III) • If the transition metal only has one charge, no Roman numeral is needed Naming Ionic Compounds with Transition Metals Given: Fe2O3 • First find the charges of the ions • Then name the cation followed by the anion – Iron(III) oxide • Try this one: Fe3P2 • the charge on iron is a 2+ • iron(II) phosphide Question Time Identify the charge on the transition metal: 1. copper (II) nitrate 2. gold (I) chromate 2+ 1+ Name the compound: 1. FeO 2. Zn(OH)2 3. CoPO4 iron (II) oxide zinc hydroxide cobalt (III) phosphate