Factors Affecting the Rate of a Chemical Reaction
advertisement

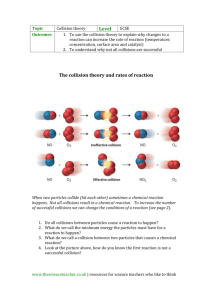
Factors Affecting the Rate of a Chemical Reaction The following events must occur before a reaction can proceed: 1. The reactant particles must collide with each other. 2. The collisions must be of enough energy to overcome the ‘energy barrier’, called the activation energy (more about this on the next slide). 3. The reactants must form new bonds to produce products. This presentation is useful as a refresher for those of you starting Unit 2 of the A-level chemistry course. Activation Energy 1 Defined as: The minimum energy required to bring about a chemical reaction. If there were no such thing as ‘activation energy’ life would be very difficult: Gasoline for your car would ignite as soon as it came into contact with air. You would burst into flames. Trees would spontaneously combust. Activation energy is why these things do not happen, there is an energy barrier so most reactions need to be ‘started off’ by putting in some energy. Activation Energy 2 Activation energy for a reaction is shown on reaction profile diagrams (do you remember these?). energy Activated intermediate Activation energy Reactants H Products Changing the Rate of a Chemical Reaction To change the rate of a reaction one or more of the following things must happen: 1. Increase the number of collisions between the reactant particles 2. Increase the energy of the collisions. 3. Decrease the activation energy. This is all very well but how can we follow the progress of a chemical reaction? Following a Chemical Reaction 1 To find the rate of a chemical reaction we must be able to follow its progress with time. We have two choices: 1. Record the increase in product concentration as the reaction progresses. 2. Record the decrease in reactant concentration as the reaction progresses. Following a Chemical Reaction 2 As an example consider the reaction between calcium carbonate and hydrochloric acid. You should already know the equation but here it is: CaCO3 + 2HCl CaCl2 + H2O + CO2 We can follow this reaction by measuring the volume of carbon dioxide produced as the reaction proceeds. Gas being collected Dilute acid Marble chips This apparatus can be used to measure the gas as it is formed. It is not the only way, look in your text book for more details. Following a Chemical Reaction 3 If you collect data for the total amount of gas produced as the reaction progresses then plot this data on a graph you should get a curve similar to that shown below. Slowing down. Volume of gas/cm3 All very well, but what does the graph tell you? Reaction finished. Reaction fastest at the beginning. The gradient or slope of the graph shows the rate of the reaction. Steeper slope = faster reaction. Time/sec. Effect of Surface Area 1 When solids take part in chemical reactions only the surface particles are exposed so they are the only ones that can collide with particles of other reactants. ‘Inner’ particles are protected and cannot collide with other particles until they become ‘exposed’. The surface particles are ‘exposed’ and can react. Effect of Surface Area 2 If we break up this ‘lump’ into smaller pieces the number of particles has not changed but the there are now more ‘surface’ particles. There is now a greater surface area with more exposed particles so more collisions can occur, hence faster reaction. Larger surface area = faster reaction. Effect of Concentration Consider the reaction between zinc and hydrochloric acid: Zn + 2HCl H2 + ZnCl2 (How could you follow the progress of this reaction? Click to find out) Acid Particles Zinc 2M hydrochloric acid 1M hydrochloric acid There are more particles of acid per unit volume in the 2M acid than there are in the 1M acid. So, there will more collisions between the acid and zinc particles in the stronger acid, giving a faster reaction. Higher concentration = faster reaction Gas Reactions The rate of reaction between gases is increased by increased pressure. In effect pressure is the gas equivalent of concentration. These two gas jars contain the same number of gas particles. The higher pressure jar has more particles per unit volume which means a higher concentration, hence faster reaction. Low pressure, particles far apart. Higher pressure, particles closer together. Higher pressure = faster reaction Effect of Temperature According to kinetic theory (do you remember this?) as the temperature increases the particles in a substance move about more quickly. Reaction at 300C Reaction at 500C As the temperature increases the number of collisions increases as well as the energy of the collisions. So temperature has a big effect on the rate of reaction. For every 100C increase the rate approximately doubles. Higher temperature = faster reaction Effect of a Catalyst 1 A catalyst is a substance that increases the speed of a reaction, without being used up. A catalyst can be ‘recovered at the end of a reaction and used again. A catalyst reduces the activation energy of a reaction. This is easier to understand with a diagram – see next slide. Effect of a Catalyst 2 Activation energy without catalyst. energy The lower activation energy in the presence of a catalyst means the reaction will be faster. More of the collisions have enough energy to react. There is a lower ‘energy barrier’. Activation energy with catalyst. Catalyst = faster reaction. More About Rate Graphs Slowing down. Volume of gas/cm3 1. Why is the reaction fastest at the beginning? Reaction finished. Reaction fastest at the beginning. 2. Why does the reaction slow down? 3. Why does the reaction eventually stop? Time/sec. 1. This is where the concentration of the reactants is highest, therefore fastest reaction. 2. As the reactants are used their concentration decreases so the rate of reaction decreases. 3. One of the reactants is used up, so there can be no further reaction. Special Note Some exothermic reactions speed up shortly after they start, this might be unexpected, but think about it! The temperature increases and this overcomes, at least to begin with, the effect of reducing the concentration. So, in some cases the reaction will speed up then slow down and eventually stop. Do not get caught out by this. Questions related to this effect are very common! Summary 1. Increasing the surface area gives a faster reaction because more particles are ‘exposed’ to the other reactant. 2. Increasing the concentration increases the rate of reaction because there are more collisions between the reactant particles. 3. Increasing the temperature increases the rate of reaction because the particles move move quickly and so collide more often and with greater energy. 4. A catalyst increases the rate of a reaction because it reduces the activation energy so more of the collisions have enough energy to react. The End Constructed by: Rob Dickens, Doha College Science Department.