The Effects of Solute on Boiling Water
advertisement

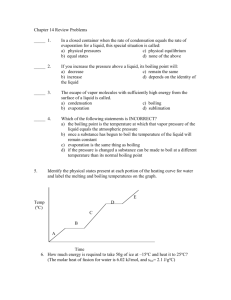
Name: Directions: Answer these questions before you begin the Activity. 1. Name two ways that the thermometer could be damaged while doing this activity. 2. Why should you wear gloves when performing this activity? 3. What safety symbols are associated with this lab? 4. What affect do you think adding salt to water will have on the boiling point of the water? 5. Explain how you convert temperatures from Celsius to Kelvin. Adding small amounts of salt to water that is being boiled to cook pasta and adding antifreeze to a radiator have a common result—increasing the boiling point. What You’ll Investigate How much can the boiling point of a solution be changed? Materials distilled water (400 mL) Epsom salt, (72 g) Bunsen burner Celsius thermometer ring stand 250-mL beaker Procedures 1. Bring 100-mL of distilled water to a gentle boil in a 250-mL beaker. Record the temperature in Table 1. 2. Dissolve 12 g of Epsom salt in 100 mL of distilled water. Bring the solution to a gentle boil and record its boiling point in Table 1. 3. Repeat steps 2, adding 12 g (24 g total) of Epsom salt. And again adding 12 grams (total of 36 g.) 4. Using Create-A-Graph Classic graph your results. (The boiling point is the dependent variable, the amount of Epsom Salt is the independent variable.) Data Table 1 The Effects of Solute on Boiling Water Grams of Solute 0 12 24 36 Boiling Point (C) Boiling Point (K) PLACE GRAPH HERE 1. Explain the difference between the boiling points of pure water and a water solution. 2. Instead of doubling the amount of NaCl in step 3, what would have been the effect of doubling the amount of water? 3. Predict what would happen if you continued to add more salt. Would your graph continue in the same pattern or eventually level off? Explain your prediction.