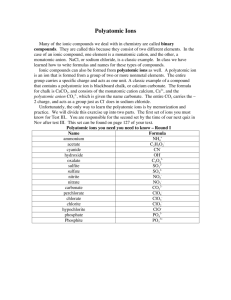
For anions
advertisement

Chapter 7 - Chemical Formulas and Chemical Compounds Hemoglobin • Where are the metals on the periodic table? • Where are the nonmetals on the periodic table? • What is a cation? An anion? • What are the charges of elements in groups 1, 2, and 13 based on their number of valence electrons? • What are the charges of elements in groups 15, 16, and 17 based on their number of valence electrons ? Warm-Up Significance of a Chemical Formula – Different for Molecular and Ionic Compounds • Molecular: The number of atoms contained in a single molecule of the compound. • Ionic: The number of ions in one formula unit, the simplest ratio of cations and anions. Chemical Formulas Molecular: Octane - C8H18 A hydrocarbon; consists of only carbon and hydrogen Ionic: Aluminum sulfate - Al2(SO4)3 The parentheses are used to indicate that there are 3 polyatomic sulfate anions in the formula unit. If there is no subscript, the value is understood to be 1. How many atoms total for each? 26 and 17, respectively Monotomic Ions Monatomic Ions Definition Steps in Creating the Formula How Do You Name It? Example Ions formed from a single atom. Main group ions form to a noble gas configuration No formulas— just ions!! For cations: The element name. For anions: The ending of the name is dropped and the ending – ide is added. Cation Ex. Ca is calcium; Ca+2 is Calcium Anion Ex. F is flourine; F-1 is flouride Examples: Naming Ions Write the symbol and the name of the ion most typically formed by Element Ion Ion name K K+ potassium cation Ca Ca2+ calcium cation S S2sulfide anion Cl Clchloride anion Binary Ionic Compounds Binary Ionic Compounds Definition Steps in Creating the Formula How Do You Name It? Example Compounds composed of two elements: a metal and a nonmetal) 1. Write the symbols and charges of the ions side by side, cation first. Al3+ O2− 2. Cross over the charges by using the absolute value of each ion’s charge as the subscript for the other ion. Make sure that the total charges now add up to zero. Al23+ O32− 3. The reason for this is so that the number of electrons that are being given by the cation can be received by the anion(s). Al2O3 1. Use the name of the cation first 2. Then use the anion name, but drop the ending and add –ide Note: You are basically just naming the monatomic ions separately and then putting them together Al2O3 Aluminum Oxide The total charge must be equal to zero. • Write the formula for the binary ionic compounds formed between the following elements: • Zinc and iodine ZnI2 • Zinc and sulfur ZnS • Sodium and sulfur Na2S • Aluminum and nitrogen AlN • Potassium and iodine KI EXAMPLE: Binary Ionic Compounds • Name the binary ionic compounds from their formula: • AgCl silver chloride • CaCl2 calcium chloride • ZnO zinc oxide • CaBr2 calcium bromide • SrF2 strontium fluoride • Al2S3 aluminum sulfide EXAMPLE: Naming Binary Ionic Compounds Transition metals Transition Metals USE THE STOCK SYSTEM OF NAMING (Roman numerals indicate the charge) Definition Steps in Creating the Formula How Do You Name It? Example D-block elements (and the metals under the staircase) When naming ionic compounds with transition metals, you MUST write the charge of the metal in Roman numerals Transition metals can have more than one charge (No formulas) The name won’t change (ex. Fe=iron= iron, no matter what the charge) Use Roman numerals after the name of the metal to indicate the charge. PbCl2 Determine the charge of the metal using the known charge of your negative ion Lead (II) chloride Pb(SO4)2 Lead (IV) Sulfate • Write the formula and give the name for the compounds that form between: • • • • • Cu2+ and BrSn2+ and FHg2+ and S2Fe2+ and O2Fe3+ and O2- CuBr2 SnF2 HgS FeO Fe2O3 copper(II)bromide tin(II)fluoride mercury(II)sulfide iron(II)oxide iron(III)oxide EXAMPLE: Stock System of Naming • give the name for the following compounds: CuO FeS CoF3 SnI4 copper(II)oxide iron(II)sulfide cobalt(III)fluoride tin(IV)iodide EXAMPLE: Stock System of Naming Compounds With Polyatomic Ions Compounds containing Polyatomic Ions Definition Steps in Creating the Formula How Do You Name It? Example Ionic compounds that use one or two positive or negative polyatomic ions to form ionic compounds Another note: Are produced by the loss of hydrogen ions (H+) from oxyacids: Sulfuric acid H2SO4 Sulfate SO42− You basically use the same steps as with putting two monatomic ions together. THE TOTAL POSITIVE CHARGE WILL BALANCE THE TOTAL NEGATIVE CHARGE. Except in this case, you won’t be changing the ending of the oxyanion (it stays –ate, or –ite.) USE TABLE 7-2!!! Al+3 bonding with (SO3)-2 Gives you Al2(SO3)3 called Aluminum Sulfate • Write the formula for: (use p. 210) • copper (II) sulfate CuSO4 • potassium sulfide K2S • potassium perchlorate KClO4 • lithium nitrate LiNO3 • calcium nitrite Ca(NO2)2 EXAMPLE: Compounds with polyatomic ions Give the names for: (use page 210) • Ca(OH)2 calcium hydroxide • FeCrO4 iron(II) chromate • KClO3 potassium chlorate • NH4OH ammonium hydroxide • KClO potassium hypochlorite EXAMPLE: Compounds with polyatomic ions • barium fluoride • cadmium oxide • tin(IV) sulfate • iron(III) nitrate • sodium hydroxide • Warm-Up • Ag2S • NH4NO3 • Cu2+ • Fe2+ • Fe3+ • O2• Br- Most Polyatomic Ions Are oxyanions, polyatomic anions that contain oxygen. Some may form different ions that contain the same elements. The most common is given the suffix –ate, the other ends in –ite. NO2− Nitrite NO3− Nitrate If More Than 2 Oxyanions Can Exist The anion with one less oxygen than the –ite anion is given the prefix hypo-. An anion with one more oxygen than the –ate anion is given the prefix per-. ClO− ClO2− ClO3− ClO4− Hypochlorite Chlorite Chlorate Perchlorate http://antoine.frostburg.edu/chem/senese/101/co mpounds/polyatomic.shtml Naming Binary Molecular Compounds Molecular compounds are covalently bonded units. Can be named in two ways: Prefix System The older system of nomenclature based on the use of prefixes to denote the number of atoms, or sometimes the number of groups of atoms, in a molecule. See Table 7-3, p. 212. Rules For the Prefix System 1. The less electronegative element is given first. It is given a prefix only if it contributes more than one atom to a molecule. 2. The second element is named by combining a. a prefix indicating the number of atoms contributed by the element, b. the root of the name of the second element, and c. The ending –ide. 3. The o or a at the end of a prefix Usually dropped when the word following the prefix begins with another vowel (monoxide, pentoxide, etc.) Thou shalt keep thy big mouth shut. • Name the following: • SO3 sulfur trioxide • ICl3 iodine trichloride • PBr5 phosphorus pentabromide EXAMPLES: Binary Molecular Compounds • Write the formulas for: • carbon tetraiodide • dinitrogen trioxide • phosphorus trichloride CI4 N2O3 PCl3 EXAMPLES: Binary Molecular Compounds Stock System Much like the Stock system for ionic compounds, but here we use oxidation numbers, which is Section 7-2. We’ll do this later. Acids (More on this in Chap. 15) Originally recognized as compounds based on their properties in solutions of water (aqueous solutions). We usually refer to solutions in water of these compounds rather than to the pure compound itself. Most Acids Used in the Laboratory Can be classified into two main types: Also see Table 7-5, p. 214. Binary Acids Consist of 2 elements, usually hydrogen and one of the halogens: HF: Hydrofluoric acid HCl: Hydrochloric acid In general, if the anion ends in –ide, the acid name will end in –ic and begin with the prefix hydro-. • Write the name for: • HI • HF • HBr • HCl hydriodic acid hydrofluoric acid hydrobromic acid hyrdrochloric acid EXAMPLE: Binary Acids Oxyacids Sulfuric acid – H2SO4 Acids that contain hydrogen, oxygen, and a third element (usually a nonmetal). If the anion ends in -ate the acid name will end as -ic. If the anion ends in -ite the acid will end as -ous. • Write formulas for the following compounds • Chloric acid HClO3 • Sulfurous acid H2SO3 • Sulfuric acid H2SO4 • Phosphoric acid H3PO4 EXAMPLE: Oxyacids Many Polyatomic Ions Are produced by the loss of hydrogen ions (H+) from oxyacids: Sulfuric acid H2SO4 Sulfate SO42− Salt An ionic compound composed of a cation and the anion from an acid. The old definition was the product of an acid and a base reacting to form a salt and water: HCl + NaOH → NaCl + H2O Some Salt Anions Retain H From The Acid Best example comes from carbonic acid, H2CO3: The anion is named by adding the word hydrogen or the prefix bi- to the anion name: HCO3− Hydrogen carbonate ion Bicarbonate ion A Salt Formed From This NaHCO3 Sodium bicarbonate (baking soda) • IF YOU HAVE A METAL IN YOUR COMPOUND, YOU WILL NEVER USE PREFIXES…ANYWHERE… EVER Reminder Binary MOLECULAR COMPOUNDS using Prefix system Molecular Compounds Definition Steps in Creating the Formula How Do You Name It? Example Molecular Compounds are covalently bonded units. You will usually be given the formula and then you can find the oxidation numbers of the rest of the atoms from there, but you would normally draw the Lewis structure Two ways: 1. The naming system is for compounds of two nonmetallic elements. 2. The first element gets a prefix if it has a subscript in the formula 3. The second element gets the –ide suffix (ending) 4. The second element ALWAYS gets a prefix. Ex. CCl4 Carbon Tetrachloride Ex. P5O2 PentaPhosphorus Dioxide Binary Acids Binary Acids Definition Steps in Creating the Formula How Do You Name It? Example Consist of 2 elements, usually hydrogen and one of the halogens Will always have one hydrogen bonded to a highly electronegative atom to form HYDRO-root of element-IC ACID Ex. HCl Hydrochloric Acid Oxyacids OXYACIDS Acids that contain hydrogen and a polyatomic ion (the polyatomic ion is what has oxygen in it…) Steps in Creating the Formula How Do You Name It? Example Will always have one hydrogen bonded to a negatively charged group-likely a polyatomic ion (ex. NO3-1) If the anion ends in -ate the acid name will end as -ic. If the anion ends in ite the acid will end as -ous. Sulfuric acid H2SO4 Sulfurous acid H2SO3 SALTS SALTS Definition Steps in Creating the Formula How Do You Name It? Example An ionic compound composed of a cation and the anion from an acid. The old definition was the product of an acid and a base reacting to form a salt and water: HCl + NaOH → NaCl + H2O The anion is named by adding the word hydrogen or the prefix bi- to the anion name: HCO3− Hydrogen carbonate ion Bicarbonate ion Ex. NaHCO3 Sodium bicarbonate (baking soda)