File - Andrea Shoger's E
advertisement

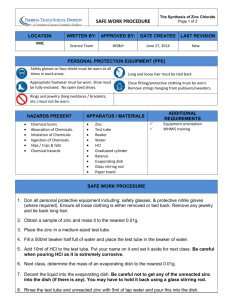
Chemical Formulas Andrea Shoger Saturday, February 08, 2014 Chem 210-5001 Lab Partner: Jessica Farace Abstract The goal of this lab was to familiarize oneself with chemical formulas and how they are obtained in experiments. By taking granular zinc and hydrochloric acid and making them react in an evaporating dish, the products of zinc chloride and hydrogen gas were formed. By setting up a steam bath and evaporating the hydrogen, the mass of chlorine was able to be obtained from the substance. Using this mass, along with the mass of zinc that was previously obtained, the balanced chemical equation was able to be formed, and the empirical formula calculated. At the conclusion of this experiment, the empirical formula was ZnCl₂ and the chemical equation that was obtained was as follows: Zn(s) + 2HCl(aq)→ZnCl₂(aq) + H₂(g). Introduction In chemistry, a sort of abbreviated notation is used to show the exact composition of chemical compounds, called chemical formulas. With this, chemical formulas can indicate how new compounds can be produced through the combination of chemical compounds, or chemical reactions. In these chemical formulas, symbols, (containing one or two letters) are used to identify the elements which are being used. These letters come from the first two letters or syllables in the element’s name. In nature, elements can be found in their molecular form. When an element is in this form, atoms of the same kind are tightly bound together, and they act as a whole unit. When a molecular element is written down, the symbol is written first, along with a subscripted number to depict how many atoms are present in each molecule. For example, F₂, or elemental fluorine, is represented by the letter F for the name of the element, as well as a subscripted 2 to explain that there are 2 fluorine atoms per molecule. Molecular compounds are those that are made up of more than on type of atom. Using the fluorine example, a compound that contains fluorine is: CuF₂, or copper difluoride. If there is no subscript in front of an element’s symbol, that means that there is only one atom of that element per molecule. Molecular formulas tell us the actual numbers of atoms in a molecule. In comparison, empirical formulas tell us of only the relative numbers of types of atoms. In empirical formulas, the subscripts are always from smallest whole-number ratios. For example, C₁₂H₃₀O₃ would simplify into C₄H₁₀O. All elements have an atomic weight, which can be found in the periodic table. The atomic weight of an element is the average atomic mass, which is expressed in what is called atomic mass units (or amu). One atomic mass unit is equal to 1.66054X10⁻²⁴grams. When obtaining the formula weight of a substance, all of the atomic weights of all atoms of the chemical formula are added together. For example, HBr, or Hydrobromic acid, has a formula weight of 80.91 amu. This is found by adding Hydrogen’s atomic weight (1.01amu) to Bromine’s (79.90amu). Similarly, if a substance is in its molecular formula, the formula’s weight is called the molecular weight. For example, Glucose has a molecular formula of C₆H₁₂O₆. To find the molecular weight, simply add the molecule’s masses together by the same process as if it were for the atomic weight. 6(12 amu) + 12(1.01 amu) + 6(16 amu) = 180.12amu. For ionic substances, however, it would be inappropriate to speak of molecules, so there should be no reference to a molecular weight or formula; although, their formula weight may be used. The example of Sodium Sulfate (Na₂SO₄) is an example of an ionic compound. This is calculated the same way as an atomic or molecular weight would be, using the amount of each substance in the compound. When new compounds are made in laboratories, the formulas of these compounds must be determined. In doing this, scientists find the percentage composition. The percentage composition is the percentage of mass of each element that is in the compound. If the formula for the compound that is being dealt with is known, the percentage of an element in a compound is found by: (#of atoms of element)(Atomic weight)/ (Formula Weight of compound) X100 Going with the example previously used with Glucose, the percent composition would be found like this: %C= 6(12amu)/(180.12amu)X100= 39.97% %H= 12(1.01amu)/(180.12amu)X100= 6.73% %O= 6(16amu)/(180.12amu)x100= 53.30% Because substances can be composed of enormous amounts of atoms, the unit called the mole was invented. This unit is used when scientists are dealing with a large number of atoms, ions, or molecules. The abbreviation for the mole is: mol. One mole refers to 6.02X10²³ (Avogadro’s number) objects. So, a mole of glucose molecules contains 6.02X10²³ C₆H₁₂O₆ molecules, and one mole of Potassium contains 6.02X10²³ K atoms. The mass of a mol of a substance is equivalent to its formula weight; this is called the molar mass. Because one H₂O molecule has a mass of 18.02amu, 1 mol of H₂O has a mass of 18.02 grams, and has 6.02X10²³ H₂O molecules. Subsequently, one K atom has a mass of 39.10amu, and 1 mol of K has a mass of 39.10 grams, and contains 6.02X10²³ K atoms. If the amount of moles was needed and all that was given was a mass, the molar mass must be divided by the given mass to obtain the amount of moles for the substance. If moles was given and mass was needed, the amount of moles must be multiplied by the molar mass to obtain the substance’s mass. To find the amount of atoms/molecules in a given substance, and all that is given is moles, multiply the amount of moles by Avogadro’s number (6.02X10²³). Similarly, if the amount of atoms or molecules is given, divide the amount of atoms by Avogadro’s number to obtain the amount of moles. The empirical formula for a substance is a ratio that tells you the relative number of atoms of each element in the given substance. For example, H₂O says that water has two hydrogen atoms for each oxygen atom in that substance. This ratio is also the same when dealing with moles. 1 mol of H₂O has 2 mol of hydrogen atoms and 1 mol of oxygen atoms. The mole allows the empirical formula for a chemical substance to be obtained. When given the percentage of each amount of an element in a substance, the empirical formula can also be obtained. For example, to calculate the empirical formula of a substance that is 50% Carbon, 25% Nitrogen, and 25% Potassium, it is safe to assume that 100g of the compound is being used, because all of the percentages add up to 100%. Therefore, the percentages of each element in the compound can directly be converted to grams. So, there would be 25g of Nitrogen, 25g Potassium, and 50g of Carbon. Next, each of the masses are divided by their atomic weight to obtain the amount of moles of each element in the sample. 50gC(1 mol C/ 12.0g C)= 4.16 mol C 25gK(1 mol K/39.0gK)= 0.64 mol K 25gN(1 mol N/14.0gN)= 1.78 mol N Finally, the obtained moles are divided by the smallest number of moles in the substance to get the simplest whole-number ratio of moles for each element (in this case, it’s 0.64 moles). Once this is calculated, the result is: KC₆N₂. It is important to remember, when calculating the empirical formula, never round up if there is a decimal of 0.7 or 0.5, and only round up if it is 0.9. Once the empirical formula is obtained, the molecular formula can be calculated if the molecular weight of the compound is known. The molecular formula is the whole-number multiple of the corresponding subscripts in its empirical formula. This multiple can be found by comparing the empirical formula’s weight with the molecular weight. Suppose an empirical formula’s weight is found to be 40.0 amu, and its determined molecular weight is 280g. By forming a ratio of these two weights (280/40=2) the molecule can be found to have 2 times the mass, and 2 times as many atoms and molecules as the empirical formula. The subscripts would then be multiplied by 2 to obtain the molecular formula. These methods were used to determine the empirical formula of Zinc Chloride in this experiment. Materials Granular zinc, evaporating dish, stirring rod, analytical balance, wire gauze, hot plate, 50mL graduated cylinder, 250mL beaker. Procedure Once an evaporating dish was obtained, it was cleaned, dried, and heated on the hot plate to evaporate all of the leftover moisture that could have remained on the dish. The dish was heated for about 5 minutes to ensure all of the moisture had left. The evaporating dish was then left to cool to room temperature, and then weighed on the analytical balance to the nearest 0.01g. Granular zinc was then obtained from the lab instructor, and about 0.5 grams was added to the evaporating dish. This was done by leaving the evaporating dish on the analytical balance, zeroing it out, and then slowly adding more and more zinc until the weight was approximately the desired mass. The evaporating dish containing the zinc was then weighed again, and the mass recorded to the nearest 0.01g. Using this information, the mass of zinc was able to be calculated. Taking the dish back to the lab station, 15 mL of 6 M HCL was slowly added to the zinc. While constantly stirring, a vigorous reaction occurred between the two substances, almost looking like an alka seltzer tablet dropped in water, with fizzing and bubbling. As this reaction was occurring, hydrogen gas was produced. If any of the zinc remained undissolved, 5mL of the acid was added in increments until all of the zinc finally did dissolve. When dealing with the zinc chloride, caution was taken, as it can be hazardous when coming In contact with skin. While the zinc was being weighed and mixed with the chloride, a steam bath was set up to boil on the hot plate. The steam bath consisted of a beaker filled with the desired amount of water that would be appropriate enough to heat the substance at a constant rate. The evaporating dish containing the zinc chloride was then placed on the steam bath, and heated. The contents of the dish were heated until almost all of the liquid disappeared. The leftover contents in the evaporating dish appeared to be pasty and waxy looking, almost like chapstick, with a very slight pinkish color. The steam bath was then removed from the hot plate, and replaced with the evaporating dish. The compound was heated for a couple more seconds, all the while keeping a close watch to make sure it did not melt. After this step, the dish was allowed to cool, and then weighed. The mass was then recorded. The dish was then gently heated again, and reweighed after it had cooled. If the weights did not agree within 0.02g, the process was to be repeated until they did. The mass of zinc chloride was then calculated. The difference between the mass of zinc and zinc chloride is the mass or chloride; and by using this information, the mass of chlorine was obtained and recorded as well. Finally, with all of the newly obtained information and known formulas, the balanced chemical equation and empirical formula was calculated. Data and calculations Table 1: Mass of Zinc Dish 55.01g Zinc 0.5042g Dish & Zinc 55.57g Mass of zinc 0.56g Table 1 includes the relevant information needed to calculate the mass of zinc. The mass of zinc was calculated by: o 55.57g dish and zinc-55.01g dish= 0.56g Table 2: chlorine and zinc chloride mass Mass 1 ZnCl₂ w/dish 56.3616g Mass 2 ZnCl₂ w/dish 56.3611g Zinc chloride mass 1.35g chlorine mass 0.79g Table 2 includes the relevant information obtained and calculated to get both the masses of zinc chloride and chlorine. The mass of zinc chloride was calculated by: o 56.3616g mass 1 – 55.01g of dish= 1.35g zinc chloride The mass of chlorine was calculated by: o 1.35g zinc chloride – 0.56g zinc= 0.79g chlorine Table 3: moles Zinc 1 mol Zn Chlorine 2.59 mol Cl Table 3 includes the moles that were calculated for zinc and chlorine. The number of moles of zinc was calculated by: o 0.56g Zn/65.39g Zn= 0.0086 mol Zn/0.0086 mol Zn = 1 mol Zn The number of moles ofchlorine was calculated by: o 0.79g Cl/35.45g Cl= 0.0223 mol Cl/0.0086 mol Zn= 2.59mol Cl Balanced chemical equation: Zn(s) + 2HCl(aq)→ZnCl₂(aq) + H₂(g) Empirical formula: ZnCl₂ Discussion of results Through the steps taken in the Procedure, the values and information obtained allowed the balanced chemical equation and empirical formula to be determined for the reaction of zinc with hydrochloric acid. Beginning with obtaining and weighing the evaporating dish, (which was 55.01g) and also weighing out an approximate amount of zinc (0.5042g) the mass of zinc was able to be obtained by subtracting the mass of the dish by the mass of zinc, and then rounding the outcome to nearest 0.01g (0.56g); as stated in Table 1. In order to get the mass of zinc chloride and chlorine, as shown in Table 2, the dish with the newly formed zinc chloride was first to be weighed two times, to ensure precision of the product. The two masses, having been 56.3616g and 56.3611g, both agreed within 0.02g, so the process did not need to be repeated. Using Mass 1, the mass of zinc chloride was obtained by subtracting the mass of the dish (55.01g) from the 56.361g of zinc chloride. The difference of these two masses provided 1.35g for the mass of zinc chloride. Once this weight was obtained, it was used to find the mass of chlorine in the substance, by subtracting the mass of zinc chloride from the mass of zinc. This mass proved to be 0.79g. Finally, with this obtained information, the empirical formula was able to be calculated. As stated before in the Introduction, the mass of one mol of a substance is equivalent to its formula weight. Because of this, by dividing the sample weight of a substance by its molar mass, the amount of moles is able to be obtained of that element in the substance. Beginning with the 0.56g of zinc, by dividing it by its molar mass found on the periodic table (65.39g zinc), 0.00086 moles of zinc was able to be calculated. Similarly, by dividing the obtained 0.79g of chloride by its molar mass of 35.45g (also found in the periodic table) the amount of moles of chloride in the substance proved to be 2.59 mol. Finally, because empirical formulas are ratios, and only tell us of the relative numbers of types of atoms in a substance, the obtained moles must be divided by the lowest number of moles to get the simplest whole-number ratio of moles for each element. Because 0.0086 mol zinc is the lowest number of moles in the substance, that is what everything will be divided by. As shown in Table 3, the amount of moles of zinc in the formula is 1, and the amount of moles for chloride is 2.59. The amount of moles calculated for each element is then used by designating the relative subscript behind its element in the empirical formula. The end result of these calculations will give the empirical formula of: ZnCl₂. Due to experimental error, however, the amount of moles of chloride in this experiment turned out to be 2.59. If this were to be correct, the formula would have had to have been multiplied by 2, in order to get rid of the decimal, making the formula: Zn₂Cl₅. However, in this experiment, it is incorrect, because the outcome was known to be ZnCl₂. This error that was made in the experiment was possibly due to the tongs accidentally touching the substance, adding some remnants of another substance from the tongs onto the solution, altogether making the mass higher than expected. Also, there could have been excess water left in the substance that did not evaporate completely, also making the mass larger, and therefore, the amount of moles larger. Having calculated this information and obtaining the empirical formula, the balanced chemical equation is then able to be formed. Using the two compounds that are given in the beginning of the Procedure, the beginnings of the equation is able to be set up as Zn(s) + HCl(aq). Zinc can be determined as a solid, because it was given in its elemental form in the experiment. HCl can be determined as aqueous, because all strong acids completely dissociate. The known outcome of the reaction is ZnCl₂(aq) (aqueous because it is a salt of Cl⁻) and H₂(g) (because hydrogen gas was produced when the reaction when it took place). Hydrogen is written with a subscripted 2, because it is a diatomic, and will always appear with 2 atoms per molecule. With this basic skeleton of the formula, the coefficients are then able to be added to balance the equation. Because there are both 2 atoms of chlorine and hydrogen on the end product of the equation, a 2 must be added in front of the HCl on the reactant side of the equation. The end product will be: Zn(s) + 2HCl(aq)→ZnCl₂(aq) + H₂(g). Conclusion The object of this experiment was to successfully obtain and calculate a balanced equation and empirical formula of a chemical reaction between granular zinc and hydrochloric acid. By first obtaining the relevant masses of the evaporating dish, zinc, zinc chloride and chlorine, the empirical formula was able to be calculated by converting the masses of zinc and chlorine to moles, and using those numbers as subscripts to their associated elements. The empirical formula that was calculated was: ZnCl₂. With the knowledge that this will be the product, along with hydrogen gas, the chemical equation was able to be formed, and balanced as: Zn(s) + 2HCl(aq)→ZnCl₂(aq) + H₂(g). References Kemp, K., Nelson, J., Stoltzfus, M. Chemistry the Central Science. 12, 55 (2012)