AP Chemistry Week 1-Practice 1
advertisement

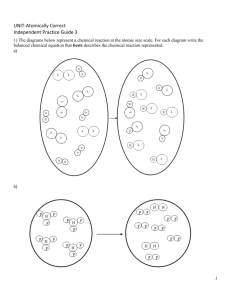
AP Chemistry 2013/2014 Unit 1 – Pre-AP Concepts Review Practice I Review Concepts: *DUE DATE: ________________________ Please complete the following problems on a separate sheet of paper. If familiar with material, these problems should be completed in approximately 45 minutes to 1 hour. Please turn in stapled to your answer sheet(s). 1. Section 1.6: Using your knowledge of metric units, convert the following: a. 160 mm to micrometers b. 43 in3 to cm3 c. 2100 mg to kg 2. Sections 2.8 to 2.9: Name the following ionic compounds: a. Sr (OH)2 b. Ca(CH3COO)2 c. (NH4)2SO3 d. Ag2Cr2O7 3. Sections 2.8 to 2.9: Write the chemical formulas for the following compounds: a. Sodium phosphate b. Iron(III)carbonate c. Aluminum hydroxide 4. Using the periodic table as your guide, predict the chemical formula and name of the compound formed by combining the following: a. Ga and F b. Li and S c. Al and I d. K and S 5. Section 3.2: Balance these equations: a. __Al(s) + __HCl(aq) yields __AlCl3(aq) + __H2(g) b. __C2H4 (g) + __O2(g) yields __CO2 (g) + __H2O(g) c. __Fe(s) + __O2(g) yields __Fe2O3(s) ) 6. Section 3.2: Write a balanced equation for the reaction that occurs when liquid methanol (CH3OH) is burned in air. 7. Section 3.2: Write a balanced combination reaction for the reaction of lithium metal and fluorine gas (be sure to include phase symbols) 8. Section 3.2: Write a balanced equation for the decomposition reaction that takes place when solid barium carbonate is heated (two products form, a solid and a gas) 9. Section 3.4: Calculate the %composition (by mass) of C,H, and O in methanol CH3OH 10. Misc.chapter 3+: How many hydrogen atoms are found in one mole of methane (CH4) molecules? 11. Section 3.4: Calculate the number of moles of ammonium chloride there are in 5.35 g of ammonium chloride. 12. Chapter 3: At STP conditions, how many atoms of gaseous sulphur is there in 1.36 L of H2S(g)? 13. Chapter 3: What is the mass of 0.5623 moles of ethanol, CH3CH2OH? 14. Section 3.7: Consider the following reaction: N2(g) + 3H2(g) yields 2NH3(g) How many grams of NH3 can be formed from 2.6 moles of N2 and 5.5 moles of H2? 15. Section 3.7: Zinc and gaseous iodine (I2(g)) react to form zinc (II) iodide (the reactants and products are all solids at room temps). a. Write a balanced chemical reaction for this process b. Suppose that 50.0 g of zinc and 50.0 g of iodine are used to form zinc(II) iodide. 1. Assuming that the reaction goes to completion, which element will be totally consumed in the formation of the zinc (II) iodide? 2. What is the limiting reagent? 3. How many grams of the product can be produced? 4. How many grams of the excess element remain unreacted? Additional Review/Practice: Empirical Formula, reaction predictions using solubility rules, and molarity