WOCCh2
advertisement

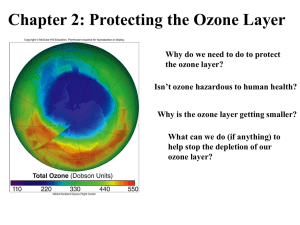
Chapter 2: Protecting the Ozone Layer Ozone Formation 3 O2 2 O3 Energy must be absorbed for this reaction Ozone is an allotropic form of oxygen. Element oxygen carbon Allotropes O2, O3 graphite, diamond, buckminister fullerenes 2.1 Atomic number (A) -The number of protons 8 O 16.00 Mass number (Z) -The sum of the protons and neutrons 2.2 The electrons in the outermost energy levels are called valence electrons. The group number (of the representative elements) on the periodic table tells you the number of valence electrons. Group 1A: 1 valence electron 1A 8A Group 3A: 3 valence electrons 2A 3A 4A 5A 6A 7A 2.2 Isotopes are two or more forms of the same element (same number of protons) whose atoms differ in number of neutrons, and hence in mass. Isotopes of carbon: C-12, C-13, C-14 also written as: 12C 13C 14C 2.2 Representing molecules with Lewis structures: Consider water, H2O: H O H 1. Find sum of valence electrons: 1 O atom x 6 valence electrons per atom = 6 + 2 H atoms x 1 valence electron per atom = 2 8 valence electrons 2. Arrange the electrons in pairs; distribute them so that the octet rule is satisfied: lone pair O H H bonding pair 2.3 Representing molecules with Lewis structures: Typical valence for selected atoms Element Typical valence 1 Classification O 2 divalent N 3 trivalent C 4 tetravalent H, X (X= F, Cl, Br, I) monovalent 2.3 Representing molecules with Lewis structures: Multiple bonds H O C C H Triple bond O Double bond Occasionally a single Lewis structure does not adequately represent the true structure of a molecule; so we use resonance forms: O O O O N N N O O O O O 2.3 The Nature of Light Low E High E Wavelength (l) = distance traveled between successive peaks (nm) Frequency (n) = number of waves passing a fixed point in one second (waves/s or 1/s or s-1 or Hz) 2.4 The Electromagnetic Spectrum The various types seem different to our senses, yet they differ only in their respective l and n. 2.4 Visible: l = 700- 400 nm ROY G BIV Decreasing wavelength Infrared (IR) : longest of the visible spectrum, heat ray absorptions cause molecules to bend and stretch Microwaves: cause molecules to rotate At short l range: UV (ultraviolet), X-rays, gamma rays 2.4 The wavelength and frequency of electromagnetic radiation are related by: C = ln where C = 3 x108 m/s (the speed of light). The energy of a photon of electromagnetic radiation is calculated by: E = h n where h = 6.63 x 10-34 J.s (Plank’s constant). Energy and frequency are directly relatedhigher frequency means higher energy. 2.5 UV radiation is of sufficient energy to cause molecular bonds to break. 2.5 2.6 The Chapman Cycle A steady state condition 2.6 Biological Effects of Ultraviolet Radiation The consequences depend primarily on: 1. The energy associated with the radiation, and 2. The sensitivity of the organism to that radiation. 2.7 How CFCs Interact with Ozone First, UV radiation breaks a carbon-halogen bond: .CClF + Cl. (free radicals) Photon (l < 220 nm) + CCl2F2 2 2.9 The chlorine radical attacks an O3 molecule: 2Cl. + 2O3 2ClO. + 2O2 Then two chlorine monoxide radicals combine: 2 ClO. ClOOCl The ClOOCl molecule then decomposes: UV photon + ClOOCl ClOO. The net reaction is: 2 O3 ClOO. + Cl. Cl. + O2 The Cl. Radicals are free to attack more O3. 3O2 2.9 As ClO. concentrations increase, ozone concentration decreases. 2.9 HCFCs are alternatives to CFCs: they decompose more readily in troposphere so they will not accumulate to the same extent in stratosphere. 2.9