Covalent naming - Fort Thomas Independent Schools
advertisement

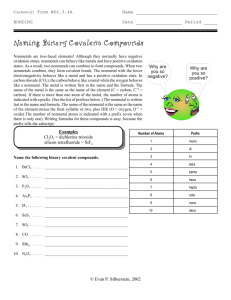
Bell ringerName or generate formulas for the
following:
GeS2
Scandium nitride
CaO
Vanadium (V) ferrocyanide
Covalent Bonds
{
Nomenclature
Covalents watch until 11:25 mark
BrightStorm
Covalent naming
Bellringer
1.
2.
3.
Identify the
following as
ionic or
covalentSodium
chloride
Cl2
4.
5.
6.
7.
Dihydrogen
monoxide
MgCl2
Germanium
Carbide
CH4
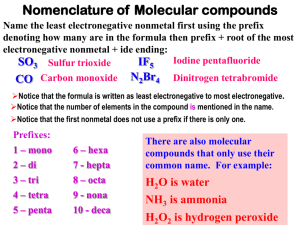
Steps to naming Covalents
1. Id metal, nonmetal (must not have a
metal)
2. Write out element names, drop
the ending off second one and replace
with –ide
3. Count how many of each element
you have.
4. Place the prefix that corresponds to
the number in front of the element name.
Special notes• Don’t ever simplify covalents
• When using tetra or penta with an
oxide drop the –a and just use -tetr or
–pent
• When using mono- with an oxide
drop the –o and just use mon• Don’t use mono- on the first word
List of prefixes# of
elements
1
2
3
4
5
prefix
mono
di
tri
tetra
penta
# of
elements
6
7
8
9
10
Prefix
hexa
hepta
octa
nona
deca
Formula
N2F4
Dinitrogen tetrafluoride
SiCl4
Silicon tetrachloride
ClF3
Chlorine trifluoride
SiO2
Silicon dioxide
Covalent
naming
Steps to writing covalent formulas
1. Id metal, nonmetal (must not have a
metal)
2. Write out symbols.
3. Read prefix, place corresponding
number in the subscript position. If it is
a mono-, leave blank don’t write the one
in the subscript position.
4. Don’t simplify!
Carbon dioxide
CO2
Phosphorous triiodide
PI3
Sulfur dichloride
SCl2
Nitrogen trifluoride
NF3
Dioxygen difluoride
O2F2
Now it’s your turn. On the
Nomenclature Worksheet, complete
all problems.
Mixed Ionic/Covalent Problems is for
homework due Friday!
Phosphorus = P4
CH4 = methane
Nitrogen = N2
Oxygen = O2