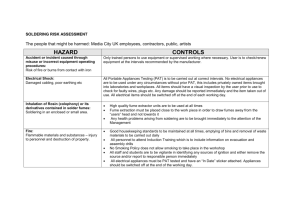
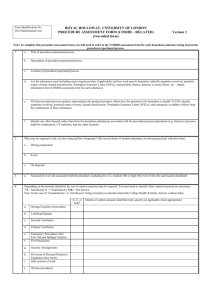
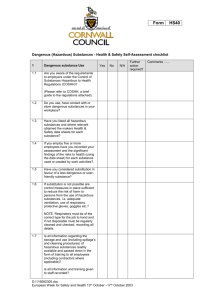
3 COSHH - Tim Woodman
advertisement

Chemical Safety – Post-Grad Induction Laboratory safety, COSHH & risk assessment Dr Tim Woodman Department of Pharmacy & Pharmacology CoSHH for Pharmacy and Pharmacology • This lecture will introduce you to CoSHH regulations, chemical safety and Good Laboratory Practice (GLP). • Dr Tim Woodman – Senior NMR Spectroscopist and Chemical Safety Officer • 9W 0.03 (NMR suite) • tw226@bath.ac.uk and ext 5555 A comic interlude… Case Study 1 UCLA researcher's death draws scrutiny to lab safety Four days after Christmas 2008, a 23-year-old research associate working in a UCLA laboratory accidentally pulled the plunger out of a syringe while conducting an experiment. The syringe contained a solution that combusts upon contact with air. The solution spilled onto Sheharbano “Sheri” Sangji’s hands and torso. Her polyester sweater burst into flames. She wasn’t wearing a lab coat; no one had told her she had to. A postdoctoral fellow from China, working nearby, tried to smother the fire with his own lab coat but didn’t think to put Sangji under an emergency shower a few feet away. By this point, deep burns covered almost half of her body. She died 18 days later. Case Study 1- findings (I) 1) Adequacy of risk syringe to transfer was questionable. conducted of this chemical. assessment. The appropriateness of using a pyrophoric liquid in the experimental procedure There was no record of any risk assessment critical operation involving a highly hazardous 2) Adequacy and record of safety training. The deceased research assistant was a fresh graduate, and had joined the research group for only three months. It was unclear how much training was given to her, or whether it included any specific and hands-on training related to handling pyrophoric chemicals and emergency response procedures. The fact that the victim ran in the direction away from the nearest emergency shower after the accident might indicate there was inadequate safety training. A family member of the deceased person alleged that no safety training was ever provided. In any case, there was no safety training record available. Case Study 1 – findings (II) 3) Proper use of fume hood and other safety devices. The victim was conducting the chemical transfer inside a fume hood, however, the sash of the fume hood was apparently raised too high to prevent the chemical from spewing onto the body of the victim. If the sash was at a lower position, or if a blast shield or a similar barrier had been placed between the body and the chemical, it might have restricted the injury to the hands and forearms. 4) Lack of protective clothing. The victim was wearing a pair of rubber gloves, which were not flame-proof, and she was not wearing a laboratory coat when the accident occurred. It also happened that she was wearing a sweater that was made of highly flammable synthetic material at the time. These factors combined to create a highly unfavourable situation when the pyrophoric material caught fire. 5) Inadequate supervision and unsatisfactory safety management. The laboratory in question was inspected two months before the accident and was found to be lacking in various aspects of chemical safety, such as improper storage of hazardous chemicals, missing first aid kits and chemical spill kits, personnel not wearing personal protective equipment such as eye protection, laboratory coats and gloves. The situation was not corrected after the due date for corrective actions. These all pointed to lack of supervision from the professor in charge of the research laboratory. Case Study 2 In January 2010, there was an explosion at Texas Tech University (TTU) that led to a graduate student losing three fingers, perforating an eye and sustaining significant burns. The incident occurred when the grad student and a colleague were working with a new energetic material, a derivative of nickel hydrazine perchlorate. A decision was taken to scale-up production of the material one hundred-fold, against lab protocol and without consulting their supervisor, with the end result being that the compound detonated. COSHH – an overview • Regulations are based on the Health and Safety at Work Act (1974), amended in 2002 • COSHH, when correctly applied, is an extremely useful approach. It will: i. Help assess risks ii. Minimise exposure to hazards iii. Help establish safe working practices • The law requires you to adequately control exposure to materials in the workplace that cause ill health • COSHH should not be seen as a pointless form-filling exercise – it is a useful process to keep you, and colleagues working around you, safe Hazardous Substances Many materials or substances used or created at work could harm your health: Pesticides Fumes/gases Powders Micro-organisms Liquids Dusts Harmful substances II • Harmful substances can be present in anything from paints and cleaners to flour dust, solder fumes, blood or waste. • Ill health caused by these substances is preventable. • Many substances can harm health but, used properly, they almost never do. • In Pharmacy and Pharmacology our main concerns are chemicals and micro-organisms. What are the hazards? Possible effects of hazardous substances (not exhaustive): Timescales: • Sometimes very rapid (dizziness, stinging eyes from fumes) • OthersAsthma may take years to develop, such as lung Cancer disease, often from repeated exposure. • Many long-term, chronic effects cannot be cured once they develop… Mesothelioma Dermatitus How is COSHH approached? •The law requires us to adequately control exposure to materials in the workplace that cause ill health. In practice this means: •Identifying which harmful substances may be present in the workplace •Deciding how workers might be exposed to them and be harmed •Looking at what measures you have in place to prevent this harm and deciding whether you are doing enough •Providing information, instruction and training •In appropriate cases, providing health surveillance How to carry out a COSHH assessment There are three main steps to follow in an assessment 1. Identify the hazards 2. Decide who might be harmed and how 3. Evaluate the risks and decide on precautions Identifying the hazards • Identification of which substances are harmful is usually achieved by reading product labels, safety data sheets (MSDS) and suppliers catalogue information. All chemicals are supplied with an MSDS, and this information is also available at several supplier websites (e.g. Sigma-Aldrich http://www.sigmaaldrich.com/safety-center.html) • If in doubt contact suppliers for further information • Consider the possibility of harmful substances being produced by your process (reaction). Sometimes two relatively benign chemicals can react to generate something far more harmful Decide who might be harmed and how • How might you be exposed? Consider the route into the body (whether the substance can be breathed in, get onto or through the skin or can even be swallowed) and the effects of exposure by each of these routes • Think of how often the substance is used and for how long • Think about anyone else who could be exposed (colleagues in the laboratory etc) • Remember cleaners, technicians, contractors and other visitors or members of the public who could be exposed • Consider what happens if controls fail Evaluate the risks and decide on precautions • Once you have carried out a risk assessment and identified which harmful substances are present, and how workers can be harmed, you need to think about preventing exposure. • Do you really need to use a particular substance, or is a safer alternative available? • Can you change the process to eliminate its use or avoid producing it? If this is not possible, you must put in place adequate control measures to reduce exposure Possible measures to reduce risks Changing the process to reduce risks • • Reduce temperature (use a lower boiling solvent?) Use pellets rather than powders Containment • Use fume cupboards/safety cabinets to minimise escape or release of harmful substances • Minimise handling, and wear appropriate personal protective equipment (PPE) Systems of work • Restrict access to those people who need to be there • Plan the storage of materials, and use appropriate containers • Plan the storage and disposal of waste Cleaning • Keep working areas clean and tidy • Have the right equipment and procedures ready to clear spillages quickly and safely • Accept that accidents will happen – be ready to deal with this Worked example You are planning to reflux a Friedel-Crafts reaction of toluene with acetyl chloride, using AlCl3 as a catalyst Firstly consider the hazards (consult MSDS, containers etc): • Toluene - Highly Flammable, harmful by inhalation • Acetyl chloride – Highly flammable, reacts violently with water, causes burns • Aluminium trichloride – Causes burns Consider safety advice: • In case if contact with eyes, wash thoroughly with water and seek medical advice • After contact with skin, wash immediately with plenty of water COSHH Who might be harmed? • You • Lab co-workers • Visitors to the lab (technicians, your supervisor, cleaners) Can you avoid the use of these chemicals? • In this case no – toluene is a reactant and solvent • Minimise the volume used (20 mL is better than 200 mL) If use cannot be avoided use suitable control measures: • Use a fume cupboard • Wear appropriate PPE (nitrile gloves, lab-coat, safety glasses) • Be prepared to deal with spills and accidents An example form… Example cont… Forms to be signed off before activity commences COSHH – final comments • These COSHH forms should be seen and initialled by TJW before work commences • Forms can be put in TJW pigeon hole, or taken to 9W 0.03 (NMR suite) for signing • Use COSHH as a positive thing, to help prepare for when things don’t go to plan Risk Assessment • COSHH assessment are used for hazardous substances • For other risks, a formal Risk Assessment should be completed • A trivial example might be – “use of a hydrogenator”, where there will be a combination of high pressure, high temperature and potentially explosive gases/liquids • Use the provided form (next slide) and if not sure – ask for help! Risk Assessment Risk Assessment How not to do it… University Health and Safety Main link to the university H and S site http://www.bath.ac.uk/hr/stayingsafewell/ P & P wiki on safety can be found here https://wiki.bath.ac.uk/display/CAF/COSHH+and+S afety+in+Pharmacy+and+Pharmacology (This includes links to the COSHH form both as PDF and editable word document) GLP Good laboratory practice – general do’s and don’ts… • Lab coats and safety glasses to be worn at all times (unless specifically advised) • No food or drink in the labs (I found breakfast cereal in one last year in a cupboard!) • Be aware of fire exits and routes, and also of safety showers and eye washers • Try to work as clean and tidy as possible – it is good practice to wash up and tidy before you go home each night • These are all examples of Good Laboratory Practice GLP Laboratory Coats Laboratory coats are a requirement in all laboratory environments, if you enter the laboratory you will be expected to wear a lab coat and safety glasses (even if you are not conducting an experiment) You should also wear appropriate clothing under your lab coat. In particular solid shoes. Sandals and open shoes will not protect your feet from chemicals or dropped equipment!!! Safety Glasses If there are people in the laboratory conducting experiments you are required to wear safety glasses at all times….. Even if you have finished your experiment and are writing in your book you still require safety glasses. “It is statistically relatively unlikely that you will throw hot acid or boiling solvent into your own eyes. It will be your colleagues / friends on the next bench…….” Contact lens wearers also need to be particularly careful. In accidents contact lenses will trap material between the lens and the eye. Note: Ordinary glass (spectacles) will meet the minimum requirements. However they offer no side protection and modern small minimalistic frames offer little general protection. Always let someone know • Homer’s got it right – make sure you report things that go wrong • You won’t get blamed, but it can help us take the appropriate action • Repeated accidents can point to failures of procedure – this can help us eliminate problems in future There is NO room for interpretation! Chemical (waste) disposal rules • Solvent waste must not go down sink – use appropriate bottles. This includes acetone used for washing glassware. • Solids can be bagged (e.g. silica). • Small samples can be disposed of in solvent waste. • Toxic wastes should be collected for special disposal (Bath University does this twice a year). • Sharps to sharps bins. • You will be told what to do in labs when you start. NMR for Pharmacy and Pharmacology (9W, 0.03) • P & P has two NMR spectrometers, as part of the Faculty’s Chemical Characterisation and Analysis Facility (CCAF) • Bruker 400 MHz Avance III for walk-up, general use, fitted with 60 position automated sample changer • Bruker 500 MHz Avance III service instrument, fitted with 60 position automated sample changer, run by Dr Woodman. This is also available to approved users when not in use by TJW • In addition there are 5 further instruments in Chemistry (but ours are better and less busy…) Bruker 400 MHz Avance III Spectrometer • Used for routine all routine spectra (i.e. initial 1H characterisation) • Automated queue system (BACS) and sample handling using Topspin (Bruker’s current NMR software) and automation via IconNMR • Fully auto-tunable probe, so most nuclei can be run from automation, without expert intervention • Used for routine 1H, 13C etc but also for 2D characterisation on final compounds • Longer experiments (over 15 minutes are queued overnight) Bruker 500 MHz Avance III Spectrometer • Run as a service by Dr Woodman • Samples submitted with form • Runs identical software to the 400 (Topspin, IconNMR), but is much more powerful • Used for all aspects of NMR - ideal for more demanding samples (such as VT, very small quantities) • Approved users can use this instrument when not in use by TJW NMR training • NMR training (for use of the 400) will be on Friday 2nd October at 2.15 pm • Please let me have you details via email (to tw226@bath.ac.uk), phone 5555 or let me know if you are unable to attend this session • Once you are happy using the 400 and have proven to be competent, training for use of the 500 is available on request • There is an NMR WIKI available which provides valuable information about NMR – search confluence for NMR or CCAF • Download the NMR sample submission form for the 500 from the WIKI or print it in the NMR room from the 400’s PC • If you feel your chemical analysis skills are weak consider attending the PA10029 undergraduate lectures on analysis – ask TJW about this