Lecture 5
advertisement

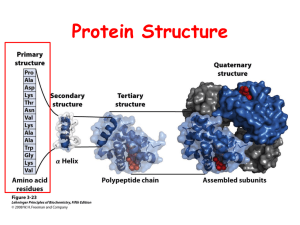
Lecture 5: Peptides – – – – – Quiz available for pickup Jahn 119 Tutoring for biochemistry (tutoring@esf.edu) More amino acid chemistry Primary structure of polypeptides Peptide synthesis Nomenclature • Glx can be Glu or Gln • Asx can be Asp or Asn • Polypeptide chains are always described from the N-terminus to the C-terminus Nomenclature • Nonhydrogen atoms of the amino acid side chain are named in sequence with the Greek alphabet Peptide bonds • Proteins are sometimes called polypeptides since they contain many peptide bonds R1 O + H3N C OH C + H H R2 O N C O- H H + H3N C R1 O R2 O C N C H H H C C O- + H 2O Structural character of amide groups • Understanding the chemical character of the amide is important since the peptide bond is an amide bond. • These characteristics are true for the amide containing amino acids as well (Asn, Gln) • Amides will not ionize but will undergo resonance -O O R C NH2 R C Resonance forms NH2 + Amide has partial charge & double bond • We can also look at the partial charge and double bond of an amide as shown below. • Since the free electrons of the N atom are tied up in forming the partial double bond, the N atom can not accept a proton (H+). • This N also has a partial positive charge which will repel protons and prevent them from binding to the nitrogen (thus no ionization). O R C NH2 Amide character in the peptide bond • Since the peptide bond is also an amide it also undergoes resonance. + H3N R1 O R2 O C N C H H H C C O- • Therefore, peptides are rigid due to resonance around the amide bond, having ≈ 40% double-bond character. • This restricts the rotation due to delocalization of electrons and overlap of the O-C-N orbitals. Amide character in the peptide bond • The double bond character results in a planar form around the peptide bond. Structural hierarchy in proteins • Primary structure (1º structure)-for a protein is the amino acid sequence of its polypeptide chain(s). • Secondary structure (2º structure)-the local spatial arrangement of a polypeptide’s backbone atoms without regard to the conformations of their side chains. • Tertiary structure (3º structure)-refers to the 3dimensional structure of an entire polypeptide (close to secondary structure). • Quaternary structure (4º structure)-The spatial arrangement of a protein’s subunits – Most protein is made up of two or more polypeptide chains (subunits) associated through noncovalent interactions. Structural hierarchy in proteins Primary structure (1º structure) of proteins • Primary structure (1º structure)-for a protein is the amino acid sequence of its polypeptide chain(s). • Amino acid sequence of a protein determines – three-dimensional conformation. – Resulting functional specificity (molecular mechanism of action) • Sequence comparisons among analogous proteins are important in comparing how proteins function and have indicated evolutionary relationships among proteins • Amino acid sequence analyses have important clinical applications because many diseases are caused by mutations that lead to an amino acid change in a protein. • Therefore, amino acid sequence analysis is an important tool for research. General approach for the analysis of the amino acid sequence of a protein • • • • • • Purify protein to homogeneity Break disulfide bonds Determine the aa composition Identify the N-terminal sequence Identify the C-terminal sequence Break the polypeptide into fragments by internal cleavage (Trypsin, chymotrypsin, pepsin, CNBr). • Determine the amino acid sequence of each fragment. • Repeat using different enzymes or CNBr. • Overlap and align fragments. Breaking disulfide bonds • • Recall that cysteine (Cys-SH HS-Cys) can convert to cystine (Cys-S-S-Cys) in the presence of air (oxidation) and will convert back if reduced. We can also prevent the formation of the disulfide bond by modifying the SH group of Cys. + H3N H H ox. -OOC C + H3N Cysteine CH2 SH red. OOC C + H3N CH2 C CH2 S-S Cystine H COO- + H3N Cysteine reactions H 2 HS CH2 CH2 OH + -OOC -mercaptoethanol C + H3N CH2 C CH2 S-S Cystine H 2 -OOC C + H3N CH2 SH Cysteine + S-CH2-CH2-OH S-CH2-CH2-OH H COO- Cysteine reactions HS CH2-CH-CH-CH2 SH OH OH -OOC + H3N CH2 SH Cysteine C + H3N H 2 -OOC C H + Dithiothreitol Dithioerythritol Cleland’s reagent + + H3N CH2 C CH2 S-S Cystine HO S HO S H COO- Cysteine reactions H ICH2COOIodoacetate + -OOC C + H3N R-group CH2 SH Cysteine H -OOC C + H3N CH2 S CH2COO- + Carboxymethylcysteine HI General approach for the analysis of the amino acid sequence of a protein • • • • • • Purify protein to homogeneity Break disulfide bonds Determine the aa composition Identify the N-terminal sequence Identify the C-terminal sequence Break the polypeptide into fragments by internal cleavage (Trypsin, chymotrypsin, pepsin, CNBr). • Determine the amino acid sequence of each fragment. • Repeat using different enzymes or CNBr. • Overlap and align fragments. N-terminus identification • Sanger’s reagent - (fluorodintrobenzene) FDNB • Dansylation - (1-dimethyl-amino-naphthalene-5-sulfonyl chloride) Dansyl Chloride • Edman degradation – Invented by Pehr Edman – Phenylisothiocyanate (PITC, Edman’s Reagent) Sanger’s reagent (fluorodintrobenzene) FDNB O 2N F FDNB The reaction with FDNB is an aromatic nucleophillic substitution reaction. O 2N .. R1 O R2 O O N C C C C H N + NO2 H H HF base H H polypeptide O O R R 1 2 H O N C C C C N NO2 H H H Sanger’s reagent will also react with other amino groups (epsilon amino group in-lysine). But only one alpha amino group will be labeled by this reagent. Aromatic amino groups are more stable than the peptide bond. Reaction with Dansyl Chloride H3C N O H3C S Cl + O Dansyl Chloride H3C R2 O O N C C C C N H H H H H HCl base H3C N .. R1 O polypeptide O H R1 O S N C C N C C O- O H R2 O H H From this we know the N-terminal amino acid and the amino acid composition but not the sequence. N-terminus identification • Sanger’s reagent - (fluorodintrobenzene) FDNB • Dansylation - (1-dimethyl-amino-naphthalene-5-sulfonyl chloride) Dansyl Chloride • Edman degradation – Invented by Pehr Edman – Phenylisothiocyanate (PITC, Edman’s Reagent) Amino acid composition of proteins • Amino acid analysis yields a protein’s amino acid composition (amounts of each amino acid in the protein). • Free amino acids can be obtained from proteins by strong acid hydrolysis: Protein 6 N HCl Amino acids 100 ºC, 24 h, in vacuo • 3 of the standard aas are lost during acid hydrolysis treatment: Asn Gln Trp Asp Amides go Glu to acids Decomposed Edman degradation I. Condensation Mild base II. Cyclization III. Conversion H+ Weak acid Possible to repeat up to 60 times using an amino acid analyzer Edman degradation • Allows the determination of the N-terminal residue identification. • Also allows us to determine the amino acid sequence of a polypeptide chain from the N-terminus inward by subjecting the polypeptide to repeated cycles of the Edman degradation and after every cycle identifying the newly liberated PTH-amino acid. Carboxy terminus identification • No reliable chemical procedure comparable to Edman degradation for the sequential end group analysis from the carboxy terminus of a polypeptide. • C-termini can be determined by hydrazine cleavage Exopeptidases cleave the ends of polypeptides • Exopeptidases recognize the ends of peptides and can be used for end group analysis • Carboxypeptidases are exopeptidases that recognize the carboxy terminal amino acids. – Carboxypeptidase A recognizes all aas except Arg/Lys/Pro; Rn-1 cannot be Pro – Carboxypeptidase B recognizes Arg/Lys; Rn-1 cannot be Pro • Aminopeptidases are exopeptidases that recognize the amino terminal amino acids. • See table 7-1 in your text. Figure 7-5a The hypothetical rate of the carboxypeptidase-catalyzed release of amino acids. (a) All bonds cleaved at the same rate. Page 165 Figure 7-5b The hypothetical rate of the carboxypeptidase-catalyzed release of amino acids. (b) Ser slow, Tyr fast, and Leu intermediate. Hydrazine cleavage R1 O R2 O N C C N C C O H H H H + polypeptide R1 O NH2-NH2 hydrazine 90 ºC, 20-100 h, in the presence of mildly acidic ion exchange resin Aminoacyl hydrazides R2 O + H3N C C NH-NH2 R0 O + H + H3N C C NH-NH2 H + + H3N C C OH Free carboxy terminal amino acid 1. Amino acids are pre- or postcolumn derivatized with dansyl chloride, Edman’s reagent, or o-phtalaldehyde (OPA) + 2-mercaptoethanol form fluorescent adducts. 2. The aas are identified are identified according to their retention times on HPLC 3. Amounts of aas present are determined fluorescent intensities. 4. Sensitive: can detect less than 1 pmol of each amino acid. OPA-amino acid analysis using reverse-phase HPLC *note OPA does not react with proline so another reagent must be used (FMOC) Specific Peptide Cleavage Reactions • Polypeptides longer than 40 to 100 residues cannot be directly sequenced. • Therefore these larger polypeptides must be cleaved into smaller fragments that are small enough to be sequenced. Endopeptidases cleave polypeptides internally • Endopeptidases catalyze the hydrolysis of internal peptide bonds • Trypsin cleaves specifically after (C-side)positively charged amino acids; Arg or Lys (basic aas) • Chymotrypsin cleaves specifically after (C-side) Trp, Phe, Tyr (aromatic aas) and slowly at Leu, Met, Asn, His. • Pepsin cleaves before (N-side) Trp, Phe, Tyr, Met, Leu and all others under acidic conditions. • Thermolysin cleaves before (N-side) Leu, Ile, Phe, Trp, Tyr, Val and sometimes for all others. • There are others. • See table 7-2 in your text. Methionine and CNBr-Internal Cleavages CH3 CH3 Cyanogen bromide S: C CH2 H CH N N CH2 Br CH2 O N +S C BrO C CH2 O N CH H O C N CH C O N CH C O H R2 H R2 O O Methionine and CNBr-Internal Cleavages Methyl thiocyanate Peptidyl homoserine lactone CH3 S C CH2 N H2O + CH2 O N CH H O N CH CH2 O C H CH2 O O + C N CH C O H R2 O Aminoacyl peptide + H3N CH C O R2 O Figure 7-7 The amino acid sequence of a polypeptide chain. To make trypsin even more versatile you can modify side chains of amino acids Lys specific reaction to hide basicity See p. 170 in your book-especially the reactions with citraconic anhydride so trypsin won’t cleave at Lys residues. Also on p. 170 conversion of Cys side group with 2bromoethylamine to make a basic group to cleave at Cys with trypsin. Lysine reactions H O + R’ C H -OOC + CH CH CH NH C CH2 2 2 2 3 + H3N Lysine aldehyde H -OOC O C CH2 CH2 CH2 CH2 N C + H3N + H Schiff base H2O + H+ Lysine reactions O H2C C O H + -OOC + CH CH CH NH C CH2 2 2 2 3 + H3N H2C C Lysine O Succinic anhydride H C CH2 CH2 CH2 CH2 N C CH2 CH2 C + H3N O O -OOC O- + 2H+ Determining primary structure of polypeptides Deduce the amino acid sequence of a simple polypeptide from the following results: A. Acid hydrolysis: (Ala2, Arg, Lys2, Met, Phe, Ser2) B. Carboxypeptidase A: (Ala) C. Trypsin: (Ala,Arg), (Lys,Phe,Ser), (Lys), (Ala, Met, Ser) D. CNBr: (Ala, Arg, Lys2, Met, Phe, Ser), (Ala, Ser) E. Thermolysin: (Ala, Arg, Ser), (Ala, Lys2, Met, Phe, Ser) Where do we start? First, from A. (acid hydrolysis) we know how many amino acids are in the polypeptide: 9 Second from B. (carboxypeptidase A), we know the last amino acid is one of the Ala. 1 - 2 - 3 - 4 - 5 - 6 - 7 - 8 -Ala We know trypsin cleaves at the carboxy side of basic aas (Lys and Arg) Trypsin: (Ala,Arg), (Lys,Phe,Ser), (Lys), (Ala, Met, Ser), so we can rearrange the amino acids as follows: Ala-Arg, either Phe-Ser-Lys or Ser-Phe-Lys, Arg-Lys or Lys-Lys, and either Lys-(Ala, Met, Ser) or Arg-(Ala, Met, Ser). For CNBr, we got two fragments (Ala, Arg, Lys2, Met, Phe, Ser) and (Ala, Ser). We know that cleavage occurs on the carboxy side of Met. So we know that Met-(Ser-Ala) or Met-(Ala-Ser). 1 - 2 - 3 - 4 - 5 - 6 - 7 - 8 -Ala For thermolysin, we know it cleaves N-terminal to Ile, Met, Phe, Trp, Tyr, Val. So (Ala, Arg, Ser) are before Met From trypsin: Ala-Arg, Phe-Ser-Lys or Ser-Phe-Lys, ArgLys or Lys-Lys, and either Lys-(Ala, Met, Ser) or Arg(Ala, Met, Ser). WE know that one Ala is the carboxy terminal amino acid, so Ala-Arg cannot be the carboxy terminus. Therefore, the only other possibility is the last sequence (Ala, Met, Ser) where Ala is the carboxy terminal amino acid. So the order at the carboxy terminus is basic aa-Met-SerAla or basic aa-Ser-Met-Ala For CNBr, we know that cleavage occurs on the carboxy side of Met. So, combined with the trypsin result we get basic aa-Met (Ser-Ala). 1 - 2 - 3 - 4 - 5 - basic aa - Met - Ser -Ala For thermolysin, we know it cleaves N-terminal to Ile, Met, Phe, Trp, Tyr, Val. So (Ala, Arg, Ser) are before Met or Phe. We know from the CNBr cleavage that the Met must be before Ser-Ala, so for the (Ala, Lys2, Met, Phe, Ser) Phe must be the 1st aa in this sequence. We also know that a basic aa precedes Met from the trypsin experiment. Since the only basic aas in this fragment are Lys, the order must be : Phe-Lys-Lys-Met-Ser-Ala 1 - 2 - 3 - Phe - Lys - Lys - Met - Ser -Ala Remember for thermolysin, we know it cleaves N-terminal to Ile, Met, Phe, Trp, Tyr, Val. So (Ala, Arg, Ser) are before Met or Phe. We know from the trypsin digest that Ala-Arg are in a specified order so the final sequence must be Ala-ArgSer Ala - Arg - Ser - Phe - Lys - Lys - Met - Ser -Ala