Diffusion and Osmosis
advertisement

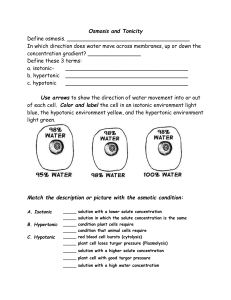
Solutions Water is considered the solvent. The substance(s) dissolved in water is / are the solute(s). Together, solvent + solute solution. Concentration is the number of solutes in each volume of solvent Osmosis The diffusion of water across a semipermeable membrane is called osmosis. In a solution, there are water molecules and dissolved particles (the solute). The more dissolved particles there are, the lower the concentration of water molecules. ANIMATION Comparing Solutions A solution may be desribed as isotonic, hypertonic or hypotonic relative to another solution These are comparisons; they require a point of reference (ie, my hair is shorter… …than it was last year). The comparison in biology is usually to the inside of a cell. Isotonic A solution is isotonic to a cell if it has the same concentration of dissolved particles as the cell. This means the water concentration is also the same. Water molecules move into and out of the cell at an equal rate in an isotonic solution. The cell size remains the same. Hypertonic A hypertonic solution has a higher concentration of dissolved particles than a cell. This means the water concentration is lower than that of the cell. Thus, water flows out of the cell – so, the cell will shrivel and eventually die. ANIMATION Hypotonic A hypotonic solution has a lower concentration of dissolved particles than a cell. Therefore the water concentration is higher than that of the cell. Thus, water diffuses into the cell – causing the cell to expand and potentially burst. Impact on Cells In an isotonic solution (center), water enters / exits red blood cells at equal rates. In a hypertonic solution (like salt water – right), water rushes out and the cell shrivels. In a hypotonic solution (like distilled water – left), water rushes in and the cell swells / bursts (lysis). Video clips: RBC in isotonic solution RBC in hypertonic solution RBC in hypotonic solution Adaptations - Plants Plant cells use the cell wall to prevent bursting. At center, the plant cell is in an isotonic solution. Water moves in / out at equal rates (no NET movement) At left, the plant cell is in a hypotonic solution. At right, the plant cell is in a hypertonic solution. Water rushes in, filling the vacuole. Water rushes out of the cell, draining the vacuole. This cell is turgid / has high turgor pressure. Video: Elodea in isotonic / hyper / hypo This is called plasmolysis. Adaptations - Protists Paramecia live in freshwater This makes paramecia hypertonic to their surroundings Water is constantly rushing into the paramecium So the paramecium uses a contractile vacuole to pump the water back out (and prevent bursting) Video: The contractile vacuole in action