9.7-9.8 Lewis Structures of Molecular Compounds, Resonance and
advertisement
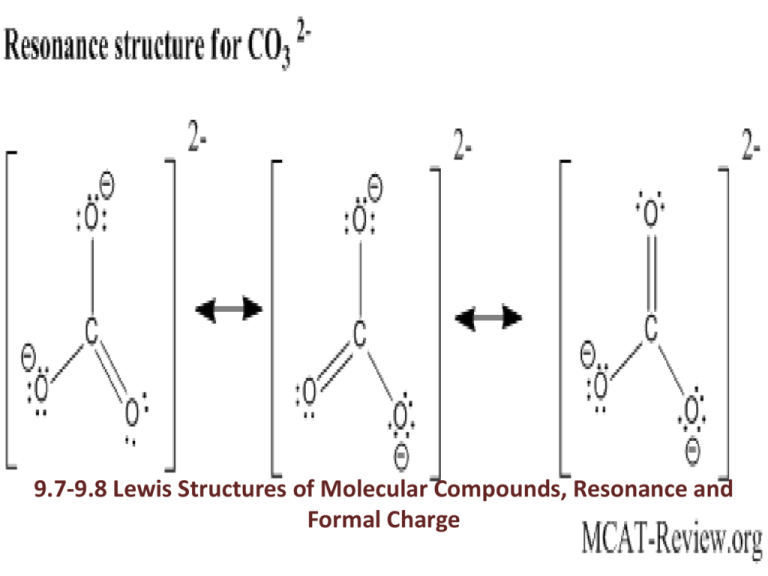
9.7-9.8 Lewis Structures of Molecular Compounds, Resonance and Formal Charge Lewis Structures of Molecular Compounds • Follow these steps: – 1.) Write the correct skeletal structure for the molecule. • Hydrogen atoms are always terminal. • Place the less electronegative atom (other than hydrogen) as the central atom. – 2.) Calculate the total number of valence electrons for the Lewis structure. -If you are writing the Lewis structure of a polyatomic ion, you must consider the charge. Also, a bracket is placed around the Lewis structure with the overall charge written as a superscript in the top right-hand corner. – 3.) Distribute the electrons among the atoms, giving octets (or duets for H) to as many atoms as possible. – 4.) If any atoms lack an octet, form a double or triple bond as necessary to give them octets. Let’s Try a Practice Problem Write the Lewis structure for CO. Write the Lewis structure for H2CO (formaldehyde). Write the Lewis structure for ClO-. Resonance • Resonance is the term used when two or more Lewis structures can be drawn for the same compound. Atoms are arranged in the same location, but have different electron arrangements. An example of a resonance structure is written below for O3 (ozone). A double headed arrow is used. Resonance structures don’t actually exist, are a convenient way to describe the actual (resonance hybrid) structure. Let’s Try a Practice Problem! Write the Lewis Structure for the NO2- ion. Include all resonance. Formal Charge • Formal charge – a fictitious charge assigned to each atom in a Lewis structure that helps to distinguish among competing Lewis structures. (The charge that each atom would have if electrons were shared equally). Here’s how to calculate formal charge: Formal charge = number of valence electrons – (number of nonbonding electrons + ½ number of bonding electrons) From the college board: Formal Charge (Continued) • Here are the rules: – 1.) The sum of all formal charges must equal 0 – 2.) The sum of all formal charges on an ion, must equal the charge of the ion. – 3.) Small (or zero) formal charges on an individual atom are better than large ones. – 4.) When formal charges can not be avoided, negative formal charge should reside on the most electronegative element. Let’s Try a Practice Problem Assign formal charges to each atom in the resonance forms of N2O. Which resonance form is likely to contribute most to the correct structure of N2O? Structures 1 (Terminal N) 2 (Central N) 3 (Terminal O) Number of v e- 5 5 5 5 5 5 6 6 6 - # of nonbonding e- -4 -2 -6 -0 -0 -0 -4 -6 -2 -½ (number of bond e-) -2 -3 -1 -4 -4 -4 -2 -1 -3 Formal Charge -1 0 -2 +1 +1 +1 0 -1 +1 Structure (B) will contribute most to the correct structure. Let’s Try Another! Draw the Lewis structure (including resonance structures) for diazomethane (CH2N2). For each resonance structure, assign formal charges to all atoms that have formal charge. Structures 1 2 3 (C) 4 (First N) (Terminal H (top)) (Terminal H (b)) Number of v e- 1 1 - # of nonbonding e- 5 1 1 4 4 5 5 5 5 -0 -0 -0 -0 -0 -2 -0 -0 -4 -2 -½ (number of bond e-) -1 -1 -1 -1 -4 -3 -4 -4 -2 -3 Formal Charge 0 0 0 0 0 -1 +1 +1 -1 0 (Terminal N 9.7-9.8 pg. 420 #’s 60, 62 & 66 Read 9.10-9.11 pgs. 409-414