Hard metal
advertisement
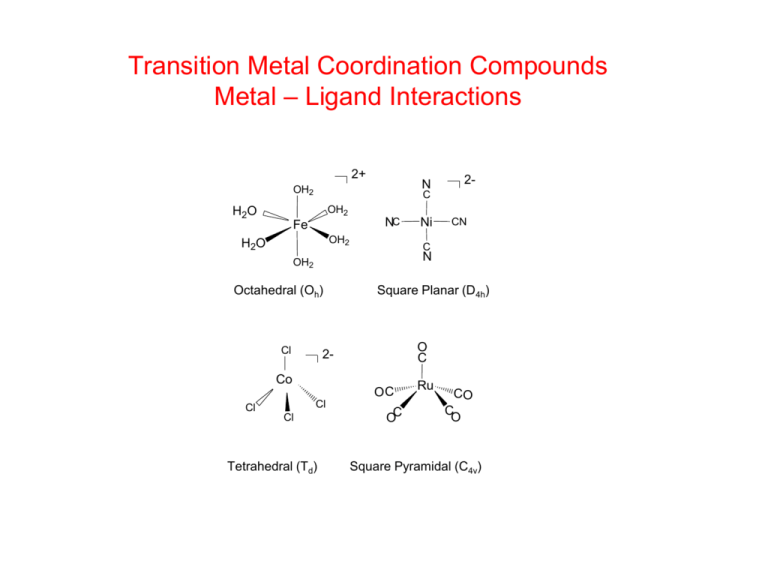
Transition Metal Coordination Compounds Metal – Ligand Interactions 2+ N OH2 OH2 H2 O Fe NC OH2 H2O Ni CN C N OH2 Octahedral (Oh) Cl Square Planar (D4h) O C 2- Co OC Cl 2- C Cl Cl Tetrahedral (Td) OC Ru CO CO Square Pyramidal (C4v) Ligands: Names and Structures Monodentate Aqua Ammine Carbonyl Chloro Bromo Cyano Acetato Bidentate / 2_ Oxo Nit ro Nitrito Thiocyanato Isothiocyanato Hydroxo Hydrido Carbonato OH2 NH3 CO_ Cl _ Br _ CN _ CH3CO2 _O _ NO2 _ _ONO _ _ NCS _ _ SCN _ OH_ H 2_ CO3 Multidentate _ 2,2 Bipyridine (bipy) N N Diethylenetriamine (dien) N N NH2 NH2 NH NH2 1,10_ P henanthroline (phen) Ethylenediamine (en) NH2 Glycinato (gly) _ _ O O NH2 O _ O2C _ O CO2 N _ N _ CO2 O2C _ Ethylenediaminet et raacetato (edta) _ C O Malonato (mal) O _ C O O Acet ylacetonato (acac) NH2 O C C O NH T riethylenet et ramine (trien) NH2 O Oxalato (ox) NH CH3 CH CH3 N N O O N N O O _ O O O T etraazacyclot etradecane (cyclam) 18-Crown-6 crown ether Chelates and Macrocycles O H2 N H2N HO2CCH2 2+ NH2 Co N H2 H2N CH2CO2H O HO2CCH2 CH2CO2H O O N FeEDTA2- EDTA NH Cu2+ O O O O O O NH O Cyclen N Fe O NH2 [Co(en)3]2+ NH O O NCH2CH2N O NH 2- O [24]crown-8 N O O O O O O N cryptand [2,2,2] Common Geometries of Transition Metal Complexes 4-Coordination M M 5-Coordination M M 6-Coordination M Tetrahedral Square Planar Trigonal bipyramidal Square Pyramidal Octahedral Tetragonal Complexation Equilibria in Water • Metallic ions in solution are surrounded by a shell (coordination sphere) of water molecules, Fe(H2O)63+, Fe(aq)3+ • Other species present in solution with available lone pairs of electrons (ligands), that have greater affinity for a metal ion than water, will displace water ligands from the inner-coordination sphere to form a complex ion or coordination complex. • Such changes are complexation equilibria and an equilibrium formation constant, Kf (stability constant) describes the ability of the ligand to bind to the metal in place of water. Stability Constants Stepwise (K1, K2, K3…) equilibrium constants, lead to an overall stability constant (β) for the complex ion. Factors contributing to metal complex stability • • • • • • Charge and Size of Metal and Ligand (electrostatic) Hard-Soft (HSAB) Nature of Metal and Ligand Chelation Macrocyclic effects Electronic Structure of Metal Solvation Effects Hard-Soft Acid-Base (HSAB) Concept • Hard metals and ligands. Hard cations have high positive charges and are not easily polarized. e.g. Fe3+. Hard ligands usually have electronegative non-polarizable donor atoms (O, N ). The metal-ligand bonding is more ionic • Soft metals and ligands. Soft cations (e.g. Hg2+, Cd2+, Cu+) have low charge densities and are easily polarized. Soft ligands usually have larger, more polarizable (S, P) donor atoms or are unsaturated molecules or ions. The metal-ligand bonding is more covalent • Borderline metals and ligands lie between hard and soft. • Hard metals like to bond to hard ligands • Soft metals like to bond to soft ligands Hard-Soft Acid-Base Classification of Metals and Ligands Hard acids Hard bases H+, Li+, Na+, K+, F-, Cl-, H2O, OH-, O2- , NO3-, Mg2+, Ca2+, Mn2+, RCO2-, ROH, RO-, phenolate Al3+, Cr3+, Co3+, Fe3+, CO3-, SO42-, PO43-, NH3, RNH2 Borderline acids Borderline bases Fe2+, Co2+, Ni2+, Cu2+, Zn2+, Sn2+ NO2-, Br-, SO32-, N3- Pb2+, Ru3+ Pyridine, imidazole, NH N Soft acids Soft acids Cu+, Ag+, Au+, Cd2+, Hg2+, Pt2+ I-, H2S, HS-, RSH, RS-, R2S, CN-, CO, R3P Stability constant trends for Fe(III) and Hg(II) halides log K1 X=F X = Cl X = Br Fe3+ + X- FeX 2+ 6.0 1.4 0.5 Hg2+ + X- HgX + 1.0 6.7 8.9 X=I 12.9 Hard metal formation constants (Kf) F Cl Br I and O >> S > Se > Soft metal formation constants (Kf) F << Cl < Br < I and O << S Se Te HSAB Concept in Geochemistry • The common ore of aluminum is alumina, Al2O3 (bauxite) while the most common ore of calcium is calcium carbonate, CaCO3 (limestone, calcite, marble). Both are hard acid - hard base combinations. Al3+ and Ca2+ are hard metals; O2- and CO32- are hard bases. • Zinc is found mostly as ZnS (wurtzite) and mercury as HgS (cinnabar). Both involve soft acid - soft base interactions. Zn2+ and Hg2+ are soft metals; S2is a soft base. Metal Chelation [Co(en)3]2+ The Chelate Effect The replacement of 2 complexed monodentate ligands by one bidentate ligands is thermodynamically favored since it generates more particles (increase in disorder) in the solution The chelate effect is an entropy effect i.e. DS is positive Thermodynamics of Complexation Enthalpy (DH) and Entropy (DS) of Complexation. The Chelate Effect Chelate Ring Size and Complex Stability 7 ox log K 1 6 O O O O ox 5 M O mal O M mal 4 O O 3 O succ O 2 M succ O 1 O Mn Fe Co Ni Cu Zn Number of chelate rings and complex stability 20 trien H2N NH NH NH2 trien dien log K 1 15 10 H2N en NH NH2 dien 5 H2N NH2 en 0 Mn Fe Co Ni Cu Zn Solvation Effects Reaction enthalpy (ΔHReact) and reaction entropy (ΔSReact) for complexation of M2+ ions by ethylenediamine, glycinate and malonate. M2+ + Ln- = ML2-n (in kJ/mol. ΔS in J/mol.K.) O O Mn2+ Co2+ Ni2+ Cu2+ Zn2+ NH2 ΔH -11.7 -28.8 -37.2 -54.3 -28.0 NH2 ΔS 12.5 16.7 23.0 22.6 16.7 NH2 ΔH -1.3 -11.7 -20.5 -25.9 -13.8 _ O ΔS 56.4 57.2 49.7 76.9 53.1 _ O ΔH 15.4 12.1 7.9 11.9 13.1 ΔS 115 113 104 148 117 _ O O Solvation Effects M2+(solv) + L (solv) → ML (solv) • Enthalpy changes (ΔHsolv) and entropy changes (ΔSsolv) arising from solvation of the metal, the ligand and the complex contribute to the overall reaction enthalpy and entropy of the complexation process. • N-donor ligands (ethylenediamine) Complexation is more enthalpy driven than entropy driven (i.e. large negative ΔH and small positive ΔS). • Mixed O- and N-donor ligand (glycinate) Less negative ΔH, and larger positive ΔS indicates that solvation entropy becomes more important with O-donors. • O-donor ligand (malonate) The small positive ΔH and large positive ΔS values indicates that the complexation is entropy driven. • O-donor ligands are more strongly solvated by water molecules. Desolvation of the O-donor ligands, prior to complexation of the metal, reduces the overall DH for the complexation reaction. i.e. energy is used to remove solvent water from the O donor atoms before they can bond to the metal. This process also adds to the reaction entropy, when the water molecules are released to the solvent. Macrocyclic complexes Macrocyclic Effect Stability constants of macrocyclic ligands are generally higher than those of their acyclic counterparts. Entropy and enthalpy changes provide driving force for the macrocyclic effect but the balance between the two is complex. Metal-ligand bonding is optimized when the size of the macrocyclic cavity and metal ion radius is closely matched. This promotes a favorable negative DH for complexation For macrocycles, there is minimal reorganization required of the polydentate ligand structure before coordination to metal. This promotes a more negative DH for complexation in macrocycles compared to corresponding acyclic open chain ligands. More extensive desolvation of ligand donor atoms may also be involved for acyclic ligands, which detracts from the overall DH for complexation.