Enzyme Regulation - Chemistry Courses: About
advertisement

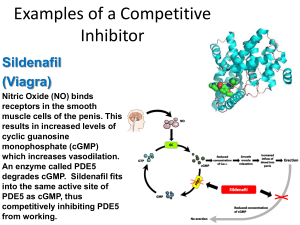
Enzyme Regulation C483 Spring 2013 Questions 1. Which statement is false about allosteric regulation? A) It is usually the mode of regulation for the last step in reaction pathways since this step produces the final product. B) Cellular response is faster with allosteric control than by controlling enzyme concentration in the cell. C) The regulation usually is important to the conservation of energy and materials in cells. D) Allosteric modulators bind non-covalently at sites other than the active site and induce conformational changes in the enzyme. 2. Which statement is false about covalent modification? A) It is reversible. B) It is slower than allosteric regulation. C) It usually uses the same enzyme for activation and inactivation. D) Phosphorylation is a common covalent modification. E) It generally involves an equilibrium between R and T states of the enzyme. 3. Allosteric modulators seldom resemble the substrate or product of the enzyme. What does this observation show? A) Modulators likely bind at a site other than the active site. B) Modulators always act as activators. C) Modulators bind non-covalently to the enzyme. D) The enzyme catalyzes more than one reaction. 4. Two curves showing the rate versus substrate concentration are shown below for an enzyme-catalyzed reaction. One curve is for the reaction in the presence of substance X. The other curve is for data in the absence of substance X. Examine the curves and tell which statement below is true. A) The catalysis shows Michaelis-Menten kinetics with or without X. B) X increases the activation energy for the catalytic reaction. C) X could be a competitive inhibitor. D) X is an activator of the enzyme. Regulation • Purpose of regulation—effects on “key” steps • Mechanisms of regulation – Control of [S]—compartmentalization, etc. – Control of [E]—transcription/translation – Both of these are limited (long term, limit of [S]) – Control of Enzyme activity • Allosteric control • Covalent Modification Allosteric Control • Same concept that we saw with hemoglobin – Applied to an enzyme, allosteric effectors control the rate of a reaction • Major Principles • Effectors bind separate site, not active site • Reversible binding • May affect Vmax or Km • Oligomeric proteins • Often found in proteins with cooperative substrate binding (see Figure to the right) Phosphofructokinase • Irreversible step in glycolysis • Regulation and logic of metabolism Regulation: Sensing the Conditions • Glucose made into chemical energy – Turn on PFK when ____________ – Turn off PFK when ________________ ATP made From ADP glucose PEP Is PEP an activator or inhibitor? Is ADP an activator or inhibitor? PFK Binding Yellow: F-1,6-bP (product) Green: ADP (product) Red: ADP (activator) Mechanism • Simple case in which substrate and activator only bind relaxed, Inhibitor only binds Tense • Not considering oligomeric aspect Allosteric • Activator – Can shift hyperbolic (as if there were no T state) • Inhibitor – Can affect Vmax or Km or both – In this graph, the inhibitor is a nonclassical competitive inhibitor— explain. Theory of Cooperativity • Concerted Model (MWC model) • Substrate binds weakly to T state (red) and strongly to R state (green) • Equilibrium is shifted to R upon binding • “Rich get richer” • Also a sequential model, but same basic idea Covalent Modification • Group may be covalently attached and removed to modulate activity • Longer range regulation (hormones) • May activate or deactivate the enzyme • Pyruvate DH – Phosphorylation • Kinase • Inactivated – Dephosphorylation • Phosphatase • Activation Answers 1. 2. 3. 4. A C A D