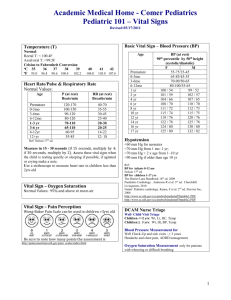
Vital Statistics Registrar Ref Manual
advertisement

National Association for Public Health Statistics and Information Systems Vital Statistics Registrar Reference Manual INTRODUCTION Purpose of Reference Manual – Contains information for new state vital statistics registrars and other staff in state vital statistics offices – Covers main vital statistics areas in one document – Primarily a reference that can be used for new registrars to quickly obtain information on vital statistics – Gives general information on major vital statistics issues and topics including a brief background and/or history – More detail can be found in other documents and information on the NAPHSIS and/or NCHS websites – Not intended to be all-inclusive on legal issues or current “hot topics” (The NAPHSIS website and the NAPHSIS LinkedIn group are good sources of information on new vital statistics issues) Vital Statistics Registrar Ref Manual 2 INTRODUCTION Organization of Manual – Organized in sections that can be accessed as needed – Slide four lists the sections and indicates where each section starts – Sections are intended to stand alone and some information may be repeated for clarity – PowerPoint format allows major points to be accessed quickly – Not meant to be read in one sitting – Most new vital statistics registrars will have some background in vital statistics and will not need to read all sections Vital Statistics Registrar Ref Manual 3 OUTLINE OF COURSE SECTIONS 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. History of Vital Statistics in the United States (slides 5-29) NAPHSIS (slides 30-53) Current NAPHSIS Projects and Resources (slides 54-81) Vital Statistics Cooperative Program (slides 82-99) National Death Index (slides 100-104) Social Security Administration Contracts (slides 105-110) Model State Vital Statistics Act and Regulations (slides 111-124) US Standard Certificates & Reports (slides 125-145) Resources for 2003 Standard Certificates (slides 146-158) Vital Record Topics (slides 159-192) Statistical Data from Vital Records (slides 193-208) Cause of Death Tabulation (slides 209-222) Vital Statistics Registrar Ref Manual 4 Section 1 History of Vital Statistics in the United States Vital Statistics Registrar Ref Manual 5 HISTORY OF VITAL STATISTICS IN THE UNITED STATES Vital Registration in the United States – Constitution does not provide for registration of vital events – Process evolved as state function in US – Different from many other countries that have national systems – Goal is to accurately record all vital events as they occur – Information on vital records was originally obtained for legal purposes – Over time collection of information greatly expanded to include statistical data for public health monitoring, research and analyses Vital Statistics Registrar Ref Manual 6 HISTORY OF VITAL STATISTICS IN THE UNITED STATES Early Collection of Vital Statistics – Vital registration began in churches with recording of christenings, marriages, and burials – In 1632, Virginia passed a law requiring ministers to report the events in court – In 1639, Massachusetts required courts to keep records of legal events of births, deaths, and marriages – Model of reporting vital events as legal statements of fact was followed in other colonies Vital Statistics Registrar Ref Manual 7 HISTORY OF VITAL STATISTICS IN THE UNITED STATES Early Collection of Vital Statistics (cont.) – Death reporting often contained information on cause of death • Useful for studying patterns of disease when epidemics occurred • By early 1800’s larger cities were establishing boards of health to monitor epidemics • In 1839 Dr. William Farr compiled information from death records to initiate sanitary reform • Need for better vital statistics data was recognized as the relationship between cause of death and bad sanitary conditions became apparent Vital Statistics Registrar Ref Manual 8 HISTORY OF VITAL STATISTICS IN THE UNITED STATES Early Collection of Vital Statistics (cont.) – In 1842, Massachusetts adopted first state registration law in US • • • • Required central state filing of records Provided for standard forms, fees and penalties Specified types of information including causes of death Designated official responsible for filing each type of record Vital Statistics Registrar Ref Manual 9 HISTORY OF VITAL STATISTICS IN THE UNITED STATES First National Vital Statistics – First birth and death statistics published for entire US based on information collected in 1850 decennial census • Persons “born within the year" • Persons " married within the year” • “Disease, if died within the year" – Collection of birth and death statistics in census continued through 1900 – Census data were inaccurate and incomplete • People do not remember all facts to report to census takers several months after event occurred • Census counts for deaths for 1850, 1860, and 1870 about 40% short of actual number of deaths Vital Statistics Registrar Ref Manual 10 HISTORY OF VITAL STATISTICS IN THE UNITED STATES Need for Better Vital Statistics Data – Physicians began pushing for more aggressive vital registration system to monitor disease outbreaks – Some cities and a few states already had vital registration systems but used different collection methods and different forms – In 1879 Congress created the National Board of Health to promote complete and uniform registration of vital events Vital Statistics Registrar Ref Manual 11 HISTORY OF VITAL STATISTICS IN THE UNITED STATES Concept of “Registration Area" – Census could accept information from areas having vital records in satisfactory detail • First used for 1880 census for death information • Books of blank death certificates provided to physicians to complete for each death they attended • Books then collected by census takers and used to improve accuracy of death reporting – Massachusetts and New Jersey and several large cities met criteria to become part of official registration area in 1880 Vital Statistics Registrar Ref Manual 12 HISTORY OF VITAL STATISTICS IN THE UNITED STATES Concept of “Registration Area" (cont.) – Promotion of standards for death data collection • Census Bureau had difficulty tabulating death records obtained for 1880 census due to differences in collection methods, forms used, and the manner of recording data • For 1890, Census Bureau requested all states and cities with a population over 5,000 to use a standard form of death certificate • In preparation for 1900 census, intensive efforts were again made to promote use of a standard death certificate • By January 1900, 12 states adopted the standard form, and 6 other states, the District of Columbia, and 71 large cities in other states adopted the form in some manner Vital Statistics Registrar Ref Manual 13 HISTORY OF VITAL STATISTICS IN THE UNITED STATES Development of Annual System for Collection of Vital Statistics from Registration of Vital Events – 1902 act made Bureau of the Census a full-time agency of the federal government – Bureau director was authorized to annually obtain copies of records filed in vital statistics offices of states and cities with adequate birth and death registration systems – Effort to obtain counts of death as part of the decennial census was abandoned Vital Statistics Registrar Ref Manual 14 HISTORY OF VITAL STATISTICS IN THE UNITED STATES Development of Annual System for Collection of Vital Statistics from Registration of Vital Events (cont.) – Census Bureau began development of uniform system for registration of vital events • Developed model law for vital registration • Drafted standard forms • Prepared instructions for local registrars, physicians and others filing records • Prepared a system of mortality classification for statistical purposes • Developed rules of statistical practice • Established working relationships with external groups • Mentioned possibility of forming national association of registrars Vital Statistics Registrar Ref Manual 15 HISTORY OF VITAL STATISTICS IN THE UNITED STATES Development of Annual System for Collection of Vital Statistics from Registration of Vital Events (cont.) – In 1907 American Public Health Association (APHA) established Vital Statistics Section • Promoted more effective vital statistics systems • Strong support for model law for registration of births and deaths – About 1913 Census Bureau began placing agents in state health agencies • To promote vital statistics registration • To improve the quality of information on vital records Vital Statistics Registrar Ref Manual 16 HISTORY OF VITAL STATISTICS IN THE UNITED STATES Development of Annual System for Collection of Vital Statistics from Registration of Vital Events (cont.) – Census Bureau publications • • • • First annual report on mortality statistics published in 1906 Data was included for five years from 1900 to 1904 Each year treated as a separate annual report Contained details on deaths for registration area states and cities – – – – By month of death Age at death Sex Color – Cause of death Vital Statistics Registrar Ref Manual 17 HISTORY OF VITAL STATISTICS IN THE UNITED STATES Development of Annual System for Collection of Vital Statistics from Registration of Vital Events (cont.) – Census Bureau publications (cont.) • By 1914 Bureau published first table separating resident deaths from nonresident (previously only published by place where event occurred) • Birth registration area formed in 1915 – Bureau began publishing annual natality data – 1915 volume had data for 10 states & District of Colombia – Tables included data on » » » » » Month of birth Sex Color Parent nativity of white children Deaths to children under one year of age Vital Statistics Registrar Ref Manual 18 HISTORY OF VITAL STATISTICS IN THE UNITED STATES Development of Annual System for Collection of Vital Statistics from Registration of Vital Events (cont.) – Efforts to increase states in registration area • Slow growth in number of states included in birth and death registration areas • In 1924, Bureau established a committee to bring all states into registration areas by 1930 • Also in 1924, Census Notification of Birth Registration was developed to be mailed to parents by state vital statistics offices when they received birth certificates • Effort made to educate boards of health, physicians, and citizens about the need for vital statistics data for public health • Not until 1933 were all states included in registration areas for births and deaths Vital Statistics Registrar Ref Manual 19 HISTORY OF VITAL STATISTICS IN THE UNITED STATES Strengthening and Improving Vital Statistics – In 1935 Halbert L. Dunn, a physician and biometrician, became director of the Division of Vital Statistics within the Census Bureau • Division was reorganized and professional staff was increased • Field work was expanded to improve completeness and accuracy of data on original certificates • Activities between federal and state offices were coordinated to eliminate duplication of effort • Research into new fields of vital statistics was begun Vital Statistics Registrar Ref Manual 20 HISTORY OF VITAL STATISTICS IN THE UNITED STATES Strengthening and Improving Vital Statistics (cont.) – New innovations in vital statistics • Births and deaths reported by place of residence of mother or decedent starting in 1935 • Monthly reporting system to provide provisional figures on births • Series of special monographs • Expansion of annual volumes • Standardization of rates for age • Extension of tabulations by age group • More analytical and interpretive material Vital Statistics Registrar Ref Manual 21 HISTORY OF VITAL STATISTICS IN THE UNITED STATES Strengthening and Improving Vital Statistics (cont.) – Collection of national marriage and divorce data began in 1940 • Followed pattern used for births and deaths • Transcripts of marriage and divorce records collected from state vital statistics offices • Marriage and divorce data collection was suspended during World War II • In 1944 publication of occurrences by state was resumed • In 1957 the Marriage Registration Area was established with 30 states and 4 territories • In 1958 the Divorce Registration Area was established with 16 states and 1 territory Vital Statistics Registrar Ref Manual 22 HISTORY OF VITAL STATISTICS IN THE UNITED STATES Increased Need for Vital Records and Statistics – By 1930s responsibility for state vital records had largely moved from civil offices to health departments – Use of vital records for public health statistical analysis expanded – More individuals needed birth records to prove “facts” about themselves • Enactment of legislation such as Social Security and beginning of pension plans • In 1940 employment in defense industry required proof of citizenship • With World War II, legislation provided for a maternal and infant care program for dependents of service men Vital Statistics Registrar Ref Manual 23 HISTORY OF VITAL STATISTICS IN THE UNITED STATES Increased Need for Vital Records and Statistics (cont.) – Some states stopped statistical functions to prepare certified copies – Many births had never been registered • States became overwhelmed with problem of filing delayed birth records • To meet demand for delayed birth records, states implemented a variety of methods • Federal agencies were confused by the many different procedures • Pressure began to grow for greater uniformity Vital Statistics Registrar Ref Manual 24 HISTORY OF VITAL STATISTICS IN THE UNITED STATES Move to Public Health Service – Recognizing problems with the national vital statistics system, the US Budget Bureau was asked by the President to recommend improvements – In 1943, based on a report from the Association of State and Territorial Health Officers, the Budget Bureau recommended • “A national vital records office” should be established in the US Public Health Service • The new office should assume functions of the Division of Vital Statistics in the Census Bureau • State and local vital statistics responsibility should be preserved Vital Statistics Registrar Ref Manual 25 HISTORY OF VITAL STATISTICS IN THE UNITED STATES Move to Public Health Service (cont.) – The national office would work with state and local vital statistics agencies to develop a nationwide vital statistics system • Promote higher standards of performance • Promote better coordination among state and local vital statistics agencies – In 1946 the National Office of Vital Statistics was established in the Public Health Service Vital Statistics Registrar Ref Manual 26 HISTORY OF VITAL STATISTICS IN THE UNITED STATES National Center for Health Statistics – In 1960 the National Office of Vital Statistics merged with the National Health Survey to become the National Center for Health Statistics (NCHS) – NCHS was reorganized in 1963 with the Division of Vital Statistics (DVS) becoming one of 5 operating divisions • Emphasis placed on analysis versus just collection and dissemination of data • Emphasis on methodological research including registration methods for vital events • Increased relationships with states • New areas of data collection including institutional data and follow-back surveys Vital Statistics Registrar Ref Manual 27 HISTORY OF VITAL STATISTICS IN THE UNITED STATES National Center for Health Statistics (cont.) – NCHS’s mandate was codified in the Public Health Service Act in 1974 • Collect statistics on health-related subjects including vital events • Provide technical assistance to state and local areas • Conduct and support research regarding survey methods • Established National Committee on Vital and Health Statistics (NCVHS) as advisory committee to the Secretary of the Department of Health, Education, and Welfare – In 1987, NCHS became part of the Centers for Disease Control (CDC) in the Department of Health and Human Services (DHHS) Vital Statistics Registrar Ref Manual 28 HISTORY OF VITAL STATISTICS IN THE UNITED STATES National Center for Health Statistics (cont.) – Currently four major data collection programs • National Vital Statistics System (NVSS) • National Health Interview Survey (NHIS) – data on health status of US population conducted through household interviews • National Health and Nutrition Examination Survey (NHANES) – assesses health and nutritional status of US population using mobile examination centers • National Health Care Surveys – data on organizations and providers of health care (Additional information on the more recent history of vital statistics in the US is provided under specific topics in other sections of this document) Vital Statistics Registrar Ref Manual 29 Section 2 National Association for Public Health Statistics and Information Systems (NAPHSIS) Vital Statistics Registrar Ref Manual 30 NAPHSIS Introduction – Represents state and local vital records, health statistics and information system agencies – Incorporated as a nonprofit in the District of Columbia – Tax exempt under section 501(c)(3) of Internal Revenue Code – Offices in Silver Spring, Maryland – Staff • • • • • Executive Director Associate Director Several project managers and directors Administrative support staff Additional consultants as needed for special projects Vital Statistics Registrar Ref Manual 31 NAPHSIS Introduction (cont.) – Web site: www.naphsis.org • General section with information about the Association and vital statistics • Members only section with special resources such as newsletters, training and background material on issues of current interest to members, member contact lists, etc. – Mission: “NAPHSIS provides national leadership for both vital records and related information systems in order to establish and protect individual identity and improve population health.” – Vision: “An accurate, timely, and secure record of all vital events in the nation.” Vital Statistics Registrar Ref Manual 32 NAPHSIS Introduction (cont.) – Strategic plan for 2013-2016: “Establish NAPHSIS as the proactive leader for developing, protecting, and using vital records and statistics systems.” • • • • • • A. Provide national advocacy for member jurisdictions B. Develop and deliver quality products and services C. Expand and strengthen NAPHSIS identity and visibility D. Grow and diversify financial resources E. Strengthen NAPHSIS organizational effectiveness F. Stand and strengthen member involvement and strategic partnerships Vital Statistics Registrar Ref Manual 33 NAPHSIS History – Organized In 1933 as the American Association of State Registration Executives (AASRE) – Original membership • Limited to “persons In Active Executive charge of the registration of vital statistics in a state department” and persons in similar positions in Canada, Mexico, and Cuba • Many original members were physicians responsible for epidemiology and vital statistics in their agencies – Original purpose of the Association was “to study and promote all matters relating to registration of vital statistics” – Representatives from 13 states attended first meeting Vital Statistics Registrar Ref Manual 34 NAPHSIS History (cont.) – Association had many name changes over the years • 1933 American Association of State Registration Executives • 1938 American Association of State and Provincial Registration Executives • 1939 American Association of Registration Executives • 1951 American Association of Registration of Executives, Inc. • 1955 American Association of Registration Executives • 1958 American Association for Vital Records and Public Health Statistics • 1980 Association for Vital Records and Health Statistics • 1995 Association for Public Health Statistics and Information Systems • 1996 National Association for Public Health Statistics and Information Systems Vital Statistics Registrar Ref Manual 35 NAPHSIS History (cont.) – Changes to purpose of the organization • Original purpose kept in the By-Laws through 1949 • Purpose expanded 1950 – “to work for the development and maintenance of sound systems of vital records that can provide the information and services needed in the best interest of the people and their government." • In 1958 purpose included public health statistics and to become advisory to ASTHO – "to provide opportunity for discussion of and group action on problems and policies involved in the administration of vital records and public health statistics programs in the United States, its territories and possessions, and to serve as an advisory group to the Association of State and Territorial Health Officers for these programs." Vital Statistics Registrar Ref Manual 36 NAPHSIS History (cont.) – Changes to membership • In 1938 membership was expanded to include executives in charge of vital statistics in US possessions and in provincial departments in Canada, Mexico, and Cuba, and in registration areas of New York City, Baltimore, and Washington, DC • In 1946 registration areas of Boston and New Orleans were added • A category of Life Member was also added in 1946 for any member who retired after serving 20 years as a registration executive Vital Statistics Registrar Ref Manual 37 NAPHSIS History (cont.) – Changes to membership (cont.) • A number of changes were made in 1950 – Membership was expanded to include persons professionally engaged in vital records work upon election by the Executive Board – The concept of Governing Council was established with members being the person in active executive charge of the vital records system in a state or territory of the US, the District of Columbia, registration areas of Boston, New York, Baltimore, and New Orleans, and the provinces and territories of Canada – Only members of the Governing Council could vote and hold office Vital Statistics Registrar Ref Manual 38 NAPHSIS History (cont.) – Changes to membership (cont.) • Additional changes in 1958 further defined membership – Areas with distinct programs in vital records and health statistics could have two members on the Governing Council – Persons professionally engaged in vital records or public health statistics programs in state or local health departments could become Associate Members – Honorary Life Membership was limited to retiring Governing Council members – Only Governing Council members could vote, serve as officers, and chair committees – Each state had one vote that was split in half if there were two Governing Council members • The By-Laws were later changed to allow Associate Members to serve as Members at Large on the Executive Committee Vital Statistics Registrar Ref Manual 39 NAPHSIS Modernizing the Association – In the late 1980s and early 1990s, several members of the Association began to explore the idea of creating a new image and structure • • • • Activities of the Association had expanded greatly With no staff, all work had to be done by the members There was limited funding to conduct activities While the Association was recognized as a vital records expert, it had little clout as a public health statistics advocate – Membership in the Association was primarily vital records administrators and statisticians in vital statistics programs – The Association had not had success in attracting professional statisticians in other health statistics subject areas Vital Statistics Registrar Ref Manual 40 NAPHSIS Modernizing the Association (cont.) – In 1994 the AVRHS Futures Committee suggested actions to meet current and future needs of the members • The purpose of the Association should be revised to focus away from administration of vital statistics to place more emphasis on public health statistics and information systems • The organization should be restructured to agency membership rather than individual membership and dues should be charged based on the population size of the registration area • The annual meeting program should be broadened to attract more diversified attendance and the meeting registration fee should be increased to raise revenue • The name of the Association should be changed to better reflect an image as a health statistics advocate • The Association should hire staff starting with a half time Executive Director and obtain office space Vital Statistics Registrar Ref Manual 41 NAPHSIS Modernizing the Association (cont.) – Major changes were made to the By-Laws in 1995 to implement suggestions of the Futures Committee • The name of the organization was changed to Association for Public Health Statistics and Information Systems with National added one year later • The purpose was greatly expanded as follows – “This Association will foster discussion and group action on issues involving public health statistics, public health information systems, and vital records registration. The Association will provide standards and principles for administering public health statistics, public health information systems, and vital records registration. The Association will represent the States and Territories of the United States regarding these issues, and will serve as an advisory group to the Association of State and Territorial Health Officials." Vital Statistics Registrar Ref Manual 42 NAPHSIS Modernizing the Association (cont.) – Changes made to the By-Laws in 1995 (cont.) • Membership in the Association – Changed from individual membership to Agency Membership with each agency able to designate up to four staff persons as voting members – Additional Individual Memberships could be purchased for agency staff – Category of Affiliate Membership was added as a non-voting for persons interested in furthering improvements in health statistics and for staff in vital statistics in the provinces of Canada and the states of Mexico – Honorary Life Membership could be awarded to anyone leaving the Association by majority vote of the Executive Committee Vital Statistics Registrar Ref Manual 43 NAPHSIS Modernizing the Association (cont.) – Changes made to the By-Laws in 1995 (cont.) • Dues structure – With the change to Agency Membership, the dues were set to be based on the population size of the state where the Agency was located » Intent for large population states to pay more than smaller ones » Originally 5 population size groups were recommended – Dues paid by other types of members would be set by the Executive Committee Vital Statistics Registrar Ref Manual 44 NAPHSIS Current NAPHSIS Governing Structure – Officers elected from Voting Membership • • • • • President President-elect Secretary Treasurer Serve two-year terms (Treasurer may be re-elected to a second consecutive term) – Board of Directors • Officers of the Association • Immediate Past President • Four Members at Large elected from Voting Membership Vital Statistics Registrar Ref Manual 45 NAPHSIS NAPHSIS Meetings – Annual meetings • Annual meetings were held from 1933 through 1955 • From 1956 through 1968 biennial meetings were held • In 1969, the Association went back to annual meetings which continue today – Regional meetings • Over the years, various regional structures were established in the Association and regional meetings were usually held • Formal Association sponsored regional meetings ceased for a period of time as resources became scarce, but a few states continued to meet to discuss and resolve common issues • With the addition of paid staff, regional meetings were reinitiated with most states attending the one in their area Vital Statistics Registrar Ref Manual 46 NAPHSIS NAPHSIS Awards – Halbert L. Dunn Award • Established in 1981 and presented at NAPHSIS annual meeting • Recognizes those who have made outstanding and lasting contributions to the fields of vital records and public health statistics • Honors Halbert L. Dunn, M.D., the Director of the National Office of Vital Statistics from 1946 to 1960 • Through 2012, twenty-seven people have been honored with this award Vital Statistics Registrar Ref Manual 47 NAPHSIS NAPHSIS Awards (cont.) – Triumph Awards • Established in 2006 to honor Association members who have bettered the vital records and health statistics field – Mentor Award – honors those who encourage colleagues to realize their full potential and help others achieve their best – Rising Star Award – recognizes the best new talent employed in the vital records and/or public health statistics and information systems area less than five years – Shining Star Achievement Award (added 2008) – honors an individual or team for extraordinary achievements that make a difference in the vital records and public health statistics and information systems field – Special awards such as the President's Award or other forms of recognition are occasionally presented to Association members and others to recognize special achievements or accomplishments Vital Statistics Registrar Ref Manual 48 NAPHSIS Benefits of NAPHSIS Membership – NAPHSIS provides the following services for members: • Leadership in national standard-setting in the health information arena • Communication and networking capabilities among members • Training and educational services and programs for members • An annual business meeting and educational program covering topics of current interest to the members • Liaison and representation with federal agencies, national and international organizations involved in public health statistics information systems, and vital records • Periodic information dissemination to members and other parties through the newsletter and electronic communication • Assistance to members in responding to daily challenges of managing public health statistics, information systems and vital records Vital Statistics Registrar Ref Manual 49 NAPHSIS NAPHSIS Relationship with Federal Agencies – Long-standing relationship with federal agencies • Original Constitution of Association made Chief Statistician for Vital Statistics in the US Census Bureau an ex officio member • Staff from federal agencies, particularly the national vital statistics agency, always participated in annual meetings • For many years the Association annual meeting was held with the NCHS Public Health Conference on Records and Statistics • For the past several years NAPHSIS annual meeting has been held jointly with NCHS VSCP Project Director’s Meeting • Representatives from other federal agencies such as Passport, Centers for Disease Control, Maternal and Child Health Bureau in HRSA, Social Security Administration, Department of Homeland Security, Department of Justice, etc. participate in annual meetings Vital Statistics Registrar Ref Manual 50 NAPHSIS NAPHSIS Relationship with Federal Agencies (cont.) – Participates jointly with NCHS in setting standards for collection, processing and dissemination of vital statistics in areas such as • • • • Standard certificates and reporting instructions Model Act and Regulations Training and quality control materials Improving the National Vital Statistics System – Acts as the state representative in negotiating contracts with federal agencies • • • • VSCP National Death Index (NDI) SSA Enumeration at Birth SSA Death Vital Statistics Registrar Ref Manual 51 NAPHSIS NAPHSIS Relationship with Federal Agencies (cont.) – Cooperative Agreement with NCHS • In 2007 NAPHSIS began 5-year cooperative agreement with NCHS – Called State Vital Statistics Improvement (VSI) Program – Agreement has been renewed for a second 5-year term • Focus of Cooperative Agreement – Provide technical assistance to the states for re-engineering vital records systems and implementing 2003 standard certificates – Development and promotion of standards and best practices » Standards for security of vital records and fraud prevention » Performance measures for all aspects of vital statistics offices Vital Statistics Registrar Ref Manual 52 NAPHSIS NAPHSIS Relationship with Federal Agencies (cont.) – Cooperative Agreement with NCHS (cont.) • Focus of Cooperative Agreement (cont.) – Training for vital statistics staff – Support for State and Territorial Exchange of Vital Events (STEVE) » Allows NAPHSIS to provide assistance to states for planning and installation » Pays for the year two and beyond maintenance fees (Additional information on projects supported by the Cooperative Agreement may be found in Section 3, Current NAPHSIS Projects) Vital Statistics Registrar Ref Manual 53 Section 3 Current NAPHSIS Projects and Resources Vital Statistics Registrar Ref Manual 54 CURRENT NAPHSIS PROJECTS AND RESOURCES Inter-Jurisdictional Exchange (IJE) Agreement – Provides a method for states to get vital event data for their residents whose events occur in other states – Administered by NAPHSIS – Information exchanged through IJE • Non-resident vital event information (births, deaths, fetal deaths, and induced terminations of pregnancy) sent to state of residence • Death information also sent to state of decedent’s birth for linking • For infants under one year of age at death, state of death may request a copy of birth certificate from state of birth – Agreement should be signed by all jurisdictions Vital Statistics Registrar Ref Manual 55 CURRENT NAPHSIS PROJECTS AND RESOURCES Inter-Jurisdictional Exchange (IJE) Agreement (cont.) – Agreement contents • Specifies restrictions on use of sending state’s vital records by receiving state in accordance with sending state’s legal requirements • Receiving states must agree to abide by the restrictions of sending states • Generally IJE agreement runs for five years – Records traditionally sent to receiving states in form of paper copies, computer listings, or data files – Most states are now switching to data transmission through the State and Territorial Exchange of Vital Events (STEVE) system Vital Statistics Registrar Ref Manual 56 CURRENT NAPHSIS PROJECTS AND RESOURCES STEVE (State and Territorial Exchange of Vital Events) – Web based system for exchanging vital events between states using standard IJE file layouts – Software application developed by NAPHSIS • Automates point-to-point exchange of IJE vital event (birth, death, fetal death, and induced termination of pregnancy) data in a standardized file format • Replaces practice of exchanging paper copies and computer abstracts currently used by most states • Will automatically strip off and send reportable data to NCHS according to VSCP contract requirements • Can send data to other approved state programs such as newborn hearing screening, immunization, voter registration, etc. via a system of "mailboxes" and data export tools Vital Statistics Registrar Ref Manual 57 CURRENT NAPHSIS PROJECTS AND RESOURCES STEVE (cont.) – Can create customized data file that can be used for approved research – Will eventually be required for reporting VSCP statistical data to NCHS replacing Secure Data Network (SDN) – STEVE currently being used in a number of jurisdictions • Installed in five pilot states in 2009 • All 57 jurisdictions expected to use STEVE along with NCHS • As of July 2013, 34 jurisdictions had installed STEVE with an additional 14 in progress or planning stages • In future STEVE can be expanded to include additional federal agencies and other trading partners Vital Statistics Registrar Ref Manual 58 CURRENT NAPHSIS PROJECTS AND RESOURCES STEVE (cont.) – Two software components • Transformation module – Contains logic to transform standard state flat file layouts into secure message packets configured to each state’s data exchange laws and policies – Tracks and delivers messages to secure “mailboxes" for vital records and other authorized program users • Controller module – Hosted by vendor – Central data repository of master file information collected from each trading partner – Allows NAPHSIS system administrator to manage trading partner community – Updates and new software releases "pushed" to active transformation modules Vital Statistics Registrar Ref Manual 59 CURRENT NAPHSIS PROJECTS AND RESOURCES STEVE (cont.) – To participate in STEVE state must • Sign IJE Agreement • Sign Memorandum of Agreement (MOA) with NAPHSIS agreeing to pay annual user fee and installation charges • Develop standard IJE file layouts • Agree to host STEVE software on its server behind its firewall • Provide secure connectivity from STEVE through its firewall to the Internet • Configure its data exchange and use rules within STEVE – NAPHSIS will assist states with planning and carrying out the installation Vital Statistics Registrar Ref Manual 60 CURRENT NAPHSIS PROJECTS AND RESOURCES EVVE (Electronic Verification of Vital Events) – Provides federal and state agencies with a quick, easy way to obtain information from birth or death certificates • Government agencies can – Verify contents of a paper birth certificate (name, age, citizenship, parents) – Or, request an electronic certification if they do not have a paper birth certificate • Examples of agencies using EVVE system – – – – – Motor vehicle agencies Medicaid offices Social Security Administration Office of Personnel Management Health departments Vital Statistics Registrar Ref Manual 61 CURRENT NAPHSIS PROJECTS AND RESOURCES EVVE (cont.) – Process • User agency submits electronic query to participating vital records jurisdictions • Query is sent to EVVE system via an XML message called a vital event transaction • EVVE system routes query to appropriate jurisdiction • Software installed in jurisdiction receives query and runs query against automated search system maintained in jurisdiction • Software constructs a response to query and returns response to EVVE hub • EVVE hub returns response to requesting user agency • Transaction is logged and user agency is billed for transaction Vital Statistics Registrar Ref Manual 62 CURRENT NAPHSIS PROJECTS AND RESOURCES EVVE (cont.) – Advantages of EVVE • Minimizes opportunities for network security breach • Reduces opportunity for users outside of a jurisdiction’s vital records office to “browse” records in jurisdiction’s database • Easy for government agency requesters to use system • Jurisdictions do not lose revenue • All transactions are logged so that any unusual activities can be traced back to requesting agency – NAPHSIS will assist states with planning and carrying out the installation Vital Statistics Registrar Ref Manual 63 CURRENT NAPHSIS PROJECTS AND RESOURCES Technical Assistance – Need for technical assistance was recognized as states began reengineering their vital records systems • Vital records offices faced a variety of problems – Implementation of the 2003 standard certificates – Higher expectations of data quality and timeliness – Costly reengineering projects in various state offices • Standards-based uniform systematic approach needed – Rethinking and redesign of business processes (reengineering) – Use of new technologies to support reengineering – Integrated vital records system that covers birth and death registration, certification and back office processes – Use of collaboration to decrease costs and improve chance for success Vital Statistics Registrar Ref Manual 64 CURRENT NAPHSIS PROJECTS AND RESOURCES Technical Assistance (cont.) – Partners involved in reengineering • SSA provided funds to assist states in developing electronic death registration (EDR) systems • NCHS provided funds and staff to – Facilitate the overall planning for reengineering – Develop requirements for model electronic birth (EBR) and death (EDR) registration systems – Coordinate requirements planning between birth and death registration groups Vital Statistics Registrar Ref Manual 65 CURRENT NAPHSIS PROJECTS AND RESOURCES Technical Assistance (cont.) – Reengineering Oversight Committee was established with representatives from NAPHSIS, NCHS and SSA – Subcommittees with many additional state representatives covered • • • • • • Electronic birth registration (EBR) Electronic death registration (EDR) Reports/files/outputs Information technology Certification and point-of-sale Data analysis Vital Statistics Registrar Ref Manual 66 CURRENT NAPHSIS PROJECTS AND RESOURCES Technical Assistance (cont.) – Process included • Use case analysis – Define, analyze and document user system interactions – Determine commonalities – Determine functional requirements including business rules • Technical guidelines – Infrastructure requirements – System performance requirements – Scalability – Disaster recovery – Archival issues Vital Statistics Registrar Ref Manual 67 CURRENT NAPHSIS PROJECTS AND RESOURCES Technical Assistance (cont.) – Documents developed are available on NAPHSIS website • • • • Use case narratives and diagrams System requirements and business rules Glossary and acronyms White papers such as – Birth/death matching – Vital records authentication and risk analysis – Certification/POS ideas in reengineering • Suggested management reports – Information from a variety of states added to NAPHSIS website as states began implementation and development of RFP's Vital Statistics Registrar Ref Manual 68 CURRENT NAPHSIS PROJECTS AND RESOURCES Technical Assistance (cont.) – NAPHSIS can provide direct technical support to states • Collaborates with NCHS to identify and work with jurisdictions having problems meeting timeliness and data quality requirements of VSCP contracts • Provides assistance in implementation of EDR & EBR systems • Provides assistance for reengineering vital records systems and implementing 2003 revised certificates • Can furnish specialized consultant to work with state – Makes site visit to review current state processes & procedures – Conducts in-depth assessment of issues in state – Makes detailed recommendations for business process improvement in registration, customer service, & statistics operations Vital Statistics Registrar Ref Manual 69 CURRENT NAPHSIS PROJECTS AND RESOURCES Security Standards and Fraud Prevention – Intelligence Reform Legislation • Passed in 2004 as the Intelligence Reform and Terrorism Prevention Act (US Public Law 108-458) • Section 7211 contains minimum standards for birth certificates • Designates Secretary of Health and Human Services (HHS) to promulgate regulations • States certain requirements for birth certificates – “certification of the birth certificate by the State or local government custodian of record that issued the certificate” – “use of safety paper or an alternative, equally secure medium” – “seal of the issuing custodian of record” – “other features designed to prevent tampering, counterfeiting, or otherwise duplicating the birth certificate for fraudulent purposes” Vital Statistics Registrar Ref Manual 70 CURRENT NAPHSIS PROJECTS AND RESOURCES Security Standards and Fraud Prevention (cont.) – Intelligence Reform Legislation (cont.) • Additional requirements – – – – Proof and verification of identity for issuance purposes Standards for processing applications to prevent fraud May not require single design for all birth certificates Must accommodate differences between states in “the manner and form in which records are stored and birth certificates are produced from such records” • In 2005 NCHS began working on regulations – Sought input from SSA, Homeland Security, other federal agencies and NAPHSIS members Vital Statistics Registrar Ref Manual 71 CURRENT NAPHSIS PROJECTS AND RESOURCES Security Standards and Fraud Prevention (cont.) – Intelligence Reform Legislation (cont.) • In 2005 NCHS began working on regulations (cont.) – Established 5 workgroups to make recommendations for regulations » » » » » Minimum paper and format standards for birth certificate Standards and best practices for physical plant security Standards and best practices for issuance of certificates Minimum processing standards up to the issuance of certificates Minimum systems standards for states to qualify for grants to support computerization of vital registration systems • Draft report released by NCHS for comment in October 2005 and new draft prepared • No additional action on regulations as of July 2013 Vital Statistics Registrar Ref Manual 72 CURRENT NAPHSIS PROJECTS AND RESOURCES Security Standards and Fraud Prevention (cont.) – NAPHSIS committees continued working on security and fraud prevention guidelines – Developed guidelines based on • Model State Vital Statistics Act and Regulations • Recommendations of intelligence reform workgroups • Best practices used in various states – Security Guide covers • • • • • • Delayed birth registration Out of institution births Birth certification documents Access to vital records Issuance of a birth certification document Physical security in vital records facility Vital Statistics Registrar Ref Manual 73 CURRENT NAPHSIS PROJECTS AND RESOURCES Security Standards and Fraud Prevention (cont.) – NAPHSIS standards developed on • • • • Management of security paper Limited access to records Employee background checks Penalty for fraudulent acquisition – Additional standards in development – Available on NAPHSIS website for members Vital Statistics Registrar Ref Manual 74 CURRENT NAPHSIS PROJECTS AND RESOURCES Security Standards and Fraud Prevention (cont.) – Security coordinator position • Motion 2006–01 passed by NAPHSIS members • Recommends each jurisdiction designate a security coordinator • Job functions – Establish office security and fraud prevention standards – Work with law enforcement on issues regarding vital statistics documents – Provide training to vital records staff on security/fraud prevention issues – Implement NAPHSIS recommended security standards and guidelines Vital Statistics Registrar Ref Manual 75 CURRENT NAPHSIS PROJECTS AND RESOURCES Performance Standards – NAPHSIS developed performance measures to • • • • Assist vital records offices in assessing their performance Encourage improvement Enhance national vital statistics system Provide information for gaining support from senior management for vital records improvement – Cover all aspects of vital records office • • • • • Registration Security Issuance Data collection and quality assurance Data transmission Vital Statistics Registrar Ref Manual 76 CURRENT NAPHSIS PROJECTS AND RESOURCES Performance Standards (cont.) – Cover all aspects of vital records office (cont.) • • • • • Data analysis and dissemination Staff supervision and training Training of other persons associated with vital records Data and record preservation Policies and procedures – Piloted by 12 vital records jurisdictions • Modifications made based on input from pilot jurisdiction • Felt standards beneficial • Requested aggregate information on how they compared with other jurisdictions Vital Statistics Registrar Ref Manual 77 CURRENT NAPHSIS PROJECTS AND RESOURCES Performance Standards (cont.) – Selected measures will be used for comparison • Standards are primarily for internal state assessment • Information will be collected by NAPHSIS from all states on small set of measures every 2-3 years • Determine level of national conformance to standards • Information only released in non-identifiable aggregate form for comparison • “Report card” provided to each state showing status on these measures • Serve as a basis for improvement – A copy is available on the NAPHSIS website Vital Statistics Registrar Ref Manual 78 CURRENT NAPHSIS PROJECTS AND RESOURCES NAPHSIS Training – Offers training opportunities at regional and annual meetings – Hosts webinars for members • Information and updates on issues • Current “hot” topics • Presentations from members on best practices – Develops training materials for members – Assists states in developing training materials for data providers such as hospital clerks, funeral directors, and medical certifiers Vital Statistics Registrar Ref Manual 79 CURRENT NAPHSIS PROJECTS AND RESOURCES NAPHSIS Training (cont.) – Extensive library of training material on website • • • • • • • • • • Cause of death training Birth certificate training PowerPoint presentations from training calls Sample state training materials Links to information for vital statistics registrars List of vital and health statistics courses offered by universities Statistical measures and definitions Examples of state statistical reports Links to state web based data query systems Links to state software code sharing Vital Statistics Registrar Ref Manual 80 CURRENT NAPHSIS PROJECTS AND RESOURCES Special Member Information – Executive Director’s newsletters distributed monthly – On NAPHSIS website • Member to Member – Updates from NAPHSIS president – News from state members – Committee reports • Minutes of board meetings • Lists of contacts in state vital statistics offices – LinkedIn group • Provides a way for members to share information on vital record issues • Allows members to question other states on how to resolve problems Vital Statistics Registrar Ref Manual 81 Section 4 Vital Statistics Cooperative Program (VSCP) Vital Statistics Registrar Ref Manual 82 VITAL STATISTICS COOPERATIVE PROGRAM (VSCP) Long Term Relationship Between States and Federal Government – US has a decentralized system with states responsible for registration of vital records – Since 1933, all states have been providing birth and death information for inclusion in national statistics – In 1946 responsibility for national vital statistics was moved from the Census Bureau to the Public Health Service – In 1974, the Public Health Services Act established a mandate for NCHS to collect health statistics on a broad range of topics Vital Statistics Registrar Ref Manual 83 VITAL STATISTICS COOPERATIVE PROGRAM (VSCP) Provision of State Data to NCHS – States sent copies (usually on microfilm) of vital records to NCHS – Reimbursed at four cents per record – NCHS coded, keyed, edited, and tabulated the data – Duplicative effort by states and NCHS to process data Vital Statistics Registrar Ref Manual 84 VITAL STATISTICS COOPERATIVE PROGRAM (VSCP) Beginnings of VSCP – In 1971 Florida began sending coded data to NCHS on computer tape without funding – By 1973 six states entered into contracts with NCHS to provide birth and demographic death data coded to NCHS specifications on computer tape – NCHS would pay a portion of state costs – Formal arrangement was named the Vital Statistics Cooperative Program (VSCP) – Existing funds were not sufficient to bring all technically ready states into the VSCP Vital Statistics Registrar Ref Manual 85 VITAL STATISTICS COOPERATIVE PROGRAM (VSCP) Cooperative Health Statistics System (CHSS) – Established in 1978 to produce “comparable and uniform health information and statistics” • Emphasis placed on cooperative relationship between federal, state and local governments • Standards for collecting, processing and analyzing health data • Eliminate duplicative efforts, more efficient use of resources, and equitable cost-sharing – Vital statistics was one of seven components included in CHSS system – The existing national vital statistics system (VSCP) was the first component of CHSS to be funded Vital Statistics Registrar Ref Manual 86 VITAL STATISTICS COOPERATIVE PROGRAM (VSCP) Working Group for Completion of the VSCP – Established in 1981 to develop formula for equitable allocation of funds among states • Previously no equitable funding arrangement for states • Best negotiators got the most funds – Members from AVRHS (now NAPHSIS) and NCHS – Formula developed by Working Group attempted to determine “true cost" of providing data • Included costs for tasks at state/local areas necessary for producing vital statistics • Presented a rationale for “federal share” of costs – Formula used to distribute VSCP funds beginning with fiscal year 1983 Vital Statistics Registrar Ref Manual 87 VITAL STATISTICS COOPERATIVE PROGRAM (VSCP) Working Group to Review the VSCP Cost Formula – Established in 1986 – Members from AVRHS and NCHS – Working Group recommendations included • Updating cost formula to fixed base level with annual cost-ofliving adjustments (COLA) and reduction in deliverables if COLAs are not achieved • Eliminating reference to “federal share" • Focus on collection of minimum basic data set of items • Requirement for states to report all minimum basic data set items to receive full funding Vital Statistics Registrar Ref Manual 88 VITAL STATISTICS COOPERATIVE PROGRAM (VSCP) Working Group to Review the VSCP Cost Formula (cont.) – Working Group recommendations included (cont.) • Requirement for new funds for implementation of new components • Clarifying NCHS rights in the data and including restrictions on use of source documents • Better procedures to handle administration of the VSCP contracts – Revised formula was implemented for fiscal year 1989 Vital Statistics Registrar Ref Manual 89 VITAL STATISTICS COOPERATIVE PROGRAM (VSCP) Second Working Group to Review VSCP Cost Formula – Established in 1992 with representatives from AVRHS and NCHS – Working Group findings • Current formula equitably distributed funds among states • No changes to formula were recommended • With increase in state automation, current formula did not adequately reflect state operations and costs • An appropriate cost and staffing model to reflect an automated vital records system should be jointly developed by NCHS and AVRHS prior to the next VSCP contract revision Vital Statistics Registrar Ref Manual 90 VITAL STATISTICS COOPERATIVE PROGRAM (VSCP) Second Working Group to Review VSCP Cost Formula (cont.) – Working Group findings (cont.) • Current formula was inadequate to reflect future vital statistics systems – Need more timely data to be used as a surveillance tool – Change way states and NCHS process vital statistics data – Goals should be set for improving timeliness of transmission of state data to NCHS – States should send data to NCHS as records received and initially processed rather than waiting for full quality control with updated records transmitted later • To reflect cooperative nature of vital statistics system, a formal MOU should be signed between NCHS and AVRHS – Minimal changes to the VSCP cost formula implemented starting with fiscal year 1995 Vital Statistics Registrar Ref Manual 91 VITAL STATISTICS COOPERATIVE PROGRAM (VSCP) Simplification of VSCP Formula – In 1998 another NCHS/NAPHSIS Working Group was established to evaluate the VSCP formula – Major changes to VSCP formula by Working Group • Greatly simplified the formula • Previous formula was overly complicated • Formula did not reflect various levels of automation used in registration areas • A formula based on cost was no longer relevant Vital Statistics Registrar Ref Manual 92 VITAL STATISTICS COOPERATIVE PROGRAM (VSCP) Simplification of VSCP Formula (cont.) – Major changes to VSCP formula by Working Group (cont.) • New distribution formula would include – An equal funding base for all registration areas – A price per record for each type of record which declined as volume increases – A price for each area’s square miles and for each licensed hospital – A salary adjustment factor which reflected differences in cost of staff in different areas – A cost of living adjustment each year – A minimum level of funding for low level registration areas Vital Statistics Registrar Ref Manual 93 VITAL STATISTICS COOPERATIVE PROGRAM (VSCP) Simplification of VSCP Formula (cont.) – VSCP contract was established as purchasing a data set rather than specific data items – The Working Group also recommended • NCHS and NAPHSIS work jointly with individual registration areas where problems exist • Registration areas have option to eliminate cause-of-death reject coding with a deduction in funding from VSCP contract • Additional funding be provided through VSCP contracts on a one-time basis to assist with implementation of the new certificate revisions – New VSCP formula implemented in 5-year contract starting in fiscal year 2000 and remained in effect with contract revisions through 2011 Vital Statistics Registrar Ref Manual 94 VITAL STATISTICS COOPERATIVE PROGRAM (VSCP) Problems with VSCP Contract – Since beginning of the VSCP, NCHS has had problems retaining adequate funding for the program • Originally NCHS cut individual data items and data sets such as marriages, divorces, and induced pregnancy terminations • With the concept of data sets implemented in 2000, NCHS began cutting the VSCP contract by decreasing the number of days per year of data purchased if funds were not available to purchase an entire year • No funding was provided to assist states with implementation of the new (2003) standard certificates at that time (Note: NCHS later provided a nominal amount to states that had revised their birth certificates before the end of 2011) Vital Statistics Registrar Ref Manual 95 VITAL STATISTICS COOPERATIVE PROGRAM (VSCP) Problems with VSCP Contract (cont.) – NAPHSIS had numerous discussions with NCHS on problems with submission of data by states • Some registration areas implemented the 2003 standard certificates, but others did not resulting in the need for NCHS to handle two different data sets • NCHS and its users wanted the new standard certificate data items from all states for analysis and research • Several states were having major problems with timely submission of data to NCHS – Improvements were needed for the entire national vital statistics system Vital Statistics Registrar Ref Manual 96 VITAL STATISTICS COOPERATIVE PROGRAM (VSCP) Current VSCP Contract – Implemented in 2012 as 5-year contract – Intent to improve the national vital statistics system – “getting from good to great” – All states designated NAPHSIS as their agent in negotiations with NCHS – NCHS provided a Statement of Work (SOW) with NAPHSIS making recommendations for revisions – Most of NAPHSIS’s recommendations were accepted Vital Statistics Registrar Ref Manual 97 VITAL STATISTICS COOPERATIVE PROGRAM (VSCP) Current VSCP Contract (cont.) – Contract includes • Shortened timelines for submission of data to NCHS – Birth final file moved from April 15 to March 1 – Death final file moved from June 15 to May 1 • Corrective Action Plan (CAP) for states not meeting contract requirements – Plan must specify actions a state will take to overcome significant problems – Examples of problems requiring CAP » Failure to use current revision of standard certificates » Failure to meet timeliness and/or data quality requirements • NCHS/NAPHSIS partnership to help states with CAP improve Vital Statistics Registrar Ref Manual 98 VITAL STATISTICS COOPERATIVE PROGRAM (VSCP) Current VSCP Contract (cont.) – Contract includes (cont.) • Requirement for all states to use 2003 standard certificates for births, deaths, and fetal deaths by January 1, 2014 • Provision for special projects for improving national vital statistics system such as – Collection of death surveillance data from pilot states – Expanding EDR use among physicians – Data quality research projects – Pricing for contract based on current volume plus a COLA each year • Adjustment to be made for changes in volume mid contract • Compensation included for early adopters of 2003 standard certificates Vital Statistics Registrar Ref Manual 99 Section 5 National Death Index (NDI) Vital Statistics Registrar Ref Manual 100 NATIONAL DEATH INDEX (NDI) Computerized Index of Death Record Information – Developed in 1980 to assist health researchers • Determine if their study subjects had died • In which state the death may have occurred – Maintained by NCHS as a resource for epidemiologists and other health researchers • Solely for statistical research purposes • May not be used for legal, administrative, or other nonresearch purposes – Death record information goes back to 1979 and death records are added annually Vital Statistics Registrar Ref Manual 101 NATIONAL DEATH INDEX (NDI) NDI Contract with States – NCHS obtains standard set of identifying information on each death from state vital statistics offices • NAPHSIS negotiated fee of $.34 per death record paid by NCHS to states • When NDI established, it was anticipated that researchers would buy death certificates from states to obtain demographic and cause of death information – In 1997, NDI Plus was added to allow researchers to obtain coded cause of death and demographic information • With NDI Plus, additional revenues are distributed to each state • A formula based on actual number of true NDI matches occurring for that state is used to determine amount (this amount may vary greatly from year to year) Vital Statistics Registrar Ref Manual 102 NATIONAL DEATH INDEX (NDI) Process for Use of NDI – Researchers submit application for review and approval – NAPHSIS members serve on application review panel – Once approved, researchers submit files of study participants to NDI for matching using various algorithms Information Researchers Obtain – Indication if their study subjects have died, date of death, state of death and death certificate number – Can contact the state of death and obtain a copy of death certificate following state research requirements – Can obtain coded cause of death information using the NDI Plus service for additional fees Vital Statistics Registrar Ref Manual 103 NATIONAL DEATH INDEX (NDI) Fees for Use of NDI – For 100,000 records or less • Routine NDI searches are $.15 per subject per year searched • NDI Plus charges are $.21 per subject per year when subject’s status (alive or dead) is unknown • If study subject is known to be deceased, NDI plus charges are $5.00 if researcher has no death certificate and $2.50 if researcher has death certificate – For 100,000 or more records known deceased • NDI Plus charges drop to $1.00 whether or not researcher has a death certificate • For 500,000 records or more, the fee drops to $0.05 per record – For 100,000 or more records of unknown vital status • One-time two year cap, not applicable to repeat searchers thereafter • If records exceed 2.5 million, the fee drops to $0.025 per subject per year Vital Statistics Registrar Ref Manual 104 Section 6 Social Security Administration Contracts Vital Statistics Registrar Ref Manual 105 SOCIAL SECURITY ADMINISTRATION CONTRACTS Enumeration at Birth (EAB) – Background • Parents had to wait to get birth certificate to apply for Social Security Number (SSN) for their child • Many states were slow in getting certified copies of birth certificates to parents • To improve process, SSA established EAB program to allow parents to request child’s SSN at hospital • Question was added to birth certificate for parents to indicate if they wanted the state vital statistics office to send birth notification to SSA • Implemented as pilot in 1987 and expanded to all states by 1997 • In 1997, SSA was also required by the Taxpayer Relief Act to collect the SSNs of the child’s parents which are passed to the IRS for tax administration Vital Statistics Registrar Ref Manual 106 SOCIAL SECURITY ADMINISTRATION CONTRACTS Enumeration at Birth (EAB) (cont.) – EAB Contract • Originally states sent data files to SSA usually on tape • Association negotiated contract with fee per record sent with a guaranteed minimum • Process was very slow in many states • With the implementation of Electronic Birth Records transmission of data to SSA greatly improved • To encourage states to speed up the process, SSA begin paying more for faster transmission • States are operating under a delivery order to provide data through June 30, 2014 • Negotiations are underway between NAPHSIS and SSA for the next contract period Vital Statistics Registrar Ref Manual 107 SOCIAL SECURITY ADMINISTRATION CONTRACTS Enumeration at Birth (EAB) (cont.) – Feedback to states • SSA links the birth certificate number to the child’s SSN • With permission of the parent, SSA returns the linked SSN and birth certificate number back to the state • The linked data may be used for public health programs Vital Statistics Registrar Ref Manual 108 SOCIAL SECURITY ADMINISTRATION CONTRACTS Social Security Administration Death – Background • SSA wanted death information from states to cut off benefit payments • States began sending data files of death information to SSA • Some states required that SSA not disclose state death information to other agencies • In 1993, federal legislation allowed SSA to disclose death information received from states to other federal agencies if those agencies paid benefits to individuals • As states began to implement electronic death records, SSA was an early supporter since it wanted the fact of death quickly to terminate benefits Vital Statistics Registrar Ref Manual 109 SOCIAL SECURITY ADMINISTRATION CONTRACTS Social Security Administration Death (cont.) – SSA Death Contract • NAPHSIS negotiated contract with fee per record sent with a guaranteed minimum • Originally states sent data files to SSA usually on tape • As electronic death records began to be implemented, SSA provided support to states through NAPHSIS • To get information on deaths as soon as possible, SSA worked with NAPHSIS to develop software for use with EDR systems – Allows funeral director to verify decedent’s SSN when entering death information in EDR – SSA is notified of death and terminates benefits • The current 5-year death contract began in January 2012 Vital Statistics Registrar Ref Manual 110 Section 7 Model State Vital Statistics Act and Regulations (Model Law) Vital Statistics Registrar Ref Manual 111 MODEL STATE VITAL STATISTICS ACT Purpose of Model Law – Promotes uniformity among states in • • • • Definitions Registration practices Disclosure and issuance procedures Other functions that comprise the state system of vital statistics – Helps ensure that vital records will be readily acceptable in all places as prima facie evidence of the facts recorded therein – Enhances the level of comparability of vital statistics data among states Vital Statistics Registrar Ref Manual 112 MODEL STATE VITAL STATISTICS ACT Purpose of Model Law (cont.) – Provides guidance to state vital statistics registrars and state legislators when revising their state laws • • • • • • • • • Requires registration of all vital events occurring in state Identifies agency authorized to register vital events Specifies time period for registration Specifies person responsible for registering vital event Contains penalties for failure to comply with the law Provides for compilation and/or publication of vital statistics Specifies method for funding vital statistics office States requirements for release of vital records Specifies any supporting documentation needed for registration – delayed births, out of hospital births • May provide for local registration in some states Vital Statistics Registrar Ref Manual 113 MODEL STATE VITAL STATISTICS ACT Background – States and NCHS work together to develop Model Law – Model Law updated periodically • To incorporate current social customs and practices • To provide for use of changing electronic technology • To provide guidance for security, confidentiality and disclosure of vital record information – Responsibility for revision of Model Law • Moved from Census Bureau to US Public Health Service in 1946 • Now in Division of Vital Statistics at NCHS First Model Act Developed in 1907 by Census Bureau – Covered both birth and death registration – Provided that forms include, at minimum, items recommended by the Census Bureau Vital Statistics Registrar Ref Manual 114 MODEL STATE VITAL STATISTICS ACT First Revision of Model Act – Tentatively approved in 1940 and adopted in 1942 – Act gave a statutory definition of vital statistics as " the registration, preparation, transcription, collection, compilation, and preservation of data pertaining to the dynamics of the population, in particular, data pertaining to births, deaths, marital status, and the data and facts incidental thereto." – First inclusion of marriages and divorces in model legislation pertaining to vital statistics – First provision for standard certificate of stillbirth – Declared vital records to be public records but restricted the right of public inspection Vital Statistics Registrar Ref Manual 115 MODEL STATE VITAL STATISTICS ACT 1959 Revision of the Model Act – Did not have major changes Model State Vital Statistics Regulations – First issued by NCHS in 1973 and included with the 1977 revision of the Model Act – To augment the Model Act – To standardize many administrative practices and procedures in state vital statistics offices – Recommended that both the Model Act and Regulations be considered when a state revises its statutes Vital Statistics Registrar Ref Manual 116 MODEL STATE VITAL STATISTICS ACT 1977 Revision of Model Act and Regulations – Changed from a locally oriented vital statistics system to centralized system • Centralized system in each state for collection, processing, registration, and certification of vital records • All vital events reported directly to state office of vital statistics • Placed local offices under direct control of state registrar • Gave state registrar option to direct local offices to perform vital record functions when it was in the interest of efficient and effective service Vital Statistics Registrar Ref Manual 117 MODEL STATE VITAL STATISTICS ACT 1977 Revision of Model Act and Regulations (cont.) – Changed fetal deaths to statistical reports instead of permanent official records – Recognized concerns about privacy, confidentiality, and fraudulent use of vital records and strengthened penalty provisions – Standardized many administrative practices and procedures used in vital statistics offices Vital Statistics Registrar Ref Manual 118 MODEL STATE VITAL STATISTICS ACT 1992 Revision of Model Act and Regulations – Meant to be flexible to accommodate new technologies for collection, storage, and retrieval of vital records – Specifically allowed for electronic production and transmission of vital records – Strengthened provisions concerned with confidentiality and security of vital records • Integrity of vital records should be protected through reasonable control of use of the records • Disclosure of information that can identify a person or institution named in a vital record should be restricted • Federal agencies or researchers who receive information from records should enter into agreements that protect the confidentiality of information provided Vital Statistics Registrar Ref Manual 119 MODEL STATE VITAL STATISTICS ACT 2011 Revision of Model Act and Regulations – Director of NCHS appointed a working group to develop the revision • Composed of seven persons from state vital statistics jurisdictions, one of whom was an attorney, and a former chief counsel of a city health department • Meetings began in 2009 – Multiple changes in new model law • Guidance in moving toward electronic certification and registration of vital events • Refocus away from paper-based registration and certification • More emphasis on security issues than previous model law Vital Statistics Registrar Ref Manual 120 MODEL STATE VITAL STATISTICS ACT 2011 Revision of Model Act and Regulations (cont.) – Multiple changes in new model law (cont.) • Sections placed in logical sequence that complements functions of collecting, recording and managing vital records • Introduced new terminology to accommodate electronic systems such as – A vital “report” is submitted and becomes a “vital record” when accepted for registration by the state registrar (“filing” a record is no longer used) – “Certification” (either paper or electronic) used instead of “certificate” or “certified copy” which refer to paper – “Establishment of parentage” replaces the term “legitimation” Vital Statistics Registrar Ref Manual 121 MODEL STATE VITAL STATISTICS ACT 2011 Revision of Model Act and Regulations (cont.) – Provisions of Model Law under 8 functional areas • Authorities - essentially the same as the 1992 revision • Security - new section to emphasize increased importance of this area and recent issues with expanded use of technology • Registration - significant revisions to section with additional specifications for registering births, deaths, and particularly delayed births; section on both amendments and corrections to records • Preservation - with electronic records, preservation includes safeguarding the record and also protecting and maintaining integrity of information contained in the record Vital Statistics Registrar Ref Manual 122 MODEL STATE VITAL STATISTICS ACT 2011 Revision of Model Act and Regulations (cont.) – Provisions of Model Law under 8 functional areas (cont.) • Disclosure - confidentiality was made a significant part of this section; provisions added for protection of personally identifiable information • Issuance - provisions added for security in issuance of vital records; concept of a single jurisdiction wide central database for all vital records to reduce the potential for fraud • Fees - fees established in regulations should reflect the approximate cost of providing the related services • Penalties - only minor changes; amounts were increased to reflect current generally higher penalties for similar violations Vital Statistics Registrar Ref Manual 123 MODEL STATE VITAL STATISTICS ACT 2011 Revision of Model Act and Regulations (cont.) – Current Status • NAPHSIS members passed Resolution 2011-1 endorsing the Model State Vital Statistics Act and Regulations and encouraged states to adopt the principles and practices of the Model when revising their legislation • The Model State Vital Statistics Act and Regulations is currently being reviewed by the Department of Health and Human Services Vital Statistics Registrar Ref Manual 124 Section 8 US Standard Certificates and Reports Vital Statistics Registrar Ref Manual 125 US STANDARD CERTIFICATES AND REPORTS Models for States to Use in Developing Their Records – Close collaboration between NCHS and states in development of standard certificates – Contain information for legal and administrative purposes • Serve as legal and personal identification • Provide information needed by federal, state and local government agencies for numerous social programs and administrative purposes – Source of data for national, state and local vital statistics – Standardize procedures for data preparation and processing to promote a uniform national data base – Represent minimum basic data set necessary to meet requirements of the VSCP contract Vital Statistics Registrar Ref Manual 126 US STANDARD CERTIFICATES AND REPORTS Periodic Review of Standard Certificates – Reviewed approximately every 10 to 15 years – Evaluated to ensure their intended uses at local, state, and national levels are met • Reflect changing conditions and user needs • Revise and improve quality of information collected and collection methods • Assess if current items are still needed and/or if new items should be added – Review includes • Persons involved in registration and statistical processes at all levels of government • Participation of major data users and providers • Input from national organizations and government agencies Vital Statistics Registrar Ref Manual 127 US STANDARD CERTIFICATES AND REPORTS Periodic Review of Standard Certificates (cont.) – Number of revisions • 12 revisions of the US Standard Certificate of Live Birth • 11 revisions of the US Standard Certificate of Death • 8 revisions of the US Standard Report of Fetal Death (previously stillbirth) • 4 revisions of the US Standard Certificate of Marriage • 4 revisions of the US Standard Certificate of Divorce, Dissolution of Marriage or Annulment • 2 revisions of the US Standard Report of Induced Termination of Pregnancy Vital Statistics Registrar Ref Manual 128 US STANDARD CERTIFICATES AND REPORTS Early Versions of the Standard Certificates – First standard certificates for live births and deaths were produced by Census Bureau in 1900 – Few changes were made in content in the early years – Early death certificate additions • Autopsy information in 1918 • Information on injuries from external causes of death in 1930 • Social Security Number and more detail on decedent’s place of residence in 1939 • Cause of death portion of certificate also revised in 1939 Vital Statistics Registrar Ref Manual 129 US STANDARD CERTIFICATES AND REPORTS Early Versions of the Standard Certificates (cont.) – Early birth certificate additions • Items related to stillbirth added in 1930 • More detail on mother’s residence added in 1939 • Certificate reformatted in 1949 to add section at bottom labeled “For medical and health use only” containing legitimacy, length of pregnancy and weight at birth • In 1968 multiple items added to birth certificate – – – – – Education of mother and father Dates of last normal menses, last live birth & last fetal death Prenatal care Complications of labor & complications related to pregnancy Congenital malformations and birth injuries of child • Apgar score & conditions affecting pregnancy added in 1978 Vital Statistics Registrar Ref Manual 130 US STANDARD CERTIFICATES AND REPORTS Standard Certificate/Report of Fetal Death – Became Separate Document in 1955 – Previously information gathered as part of birth certificate (called Stillbirth) – Closely followed birth certificate with addition of section for cause of death – Title changed to Report of Fetal Death in 1978 indicating it was a statistical report rather than a permanent document Vital Statistics Registrar Ref Manual 131 US STANDARD CERTIFICATES AND REPORTS Major Revision to Birth Certificate in 1989 – Hispanic identifier for the mother and father was added – Medical information was restructured to use check boxes rather than open-ended questions • Simplify completion of forms • Improve quality of reporting • Obtain specific information on complications of labor and/or delivery, obstetric procedures, method of delivery, congenital anomalies and abnormal conditions of newborn – Risk factors for the pregnancy added • Maternal use of tobacco and alcohol • Weight gain – Form greatly increased in size to have space for check boxes and new information Vital Statistics Registrar Ref Manual 132 US STANDARD CERTIFICATES AND REPORTS Death Certificate Changes in 1989 – Hispanic identifier and education were added for the decedent – Form was enlarged to provide more space for cause of death certification – Detailed instructions for completion of selected items was added to back of certificate – Included example for proper completion of medical certification in instructions – A single combined certificate was designed for use by physicians, coroners, and medical examiners (replaced three alternative certificates introduced in 1968) Vital Statistics Registrar Ref Manual 133 US STANDARD CERTIFICATES AND REPORTS Standard Certificates of Marriage and Divorce – First recommended for implementation in 1955 – Revisions were relatively minor over the years – 1989 versions of these certificates are currently in use Standard Report of Induced Termination of Pregnancy – Included as a standard report in 1978 – Statistical report only with no identifying information on woman having procedure – In 1989 revision only minor changes – In 1997 workgroup convened by Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion at CDC recommended a revised form to the states Vital Statistics Registrar Ref Manual 134 US STANDARD CERTIFICATES AND REPORTS 2003 Revision of Standard Certificates – Evaluation process for 2003 • Began with survey of state vital registration and statistics executives to determine whether revisions were needed • Consensus was birth and death certificates and fetal death report should be revised • Marriage and divorce certificates did not need revision – NCHS assembled panel of experts to evaluate 1989 standard certificates and recommend revisions • State vital registration and statistics executives • Representative of data provider and user organizations Vital Statistics Registrar Ref Manual 135 US STANDARD CERTIFICATES AND REPORTS 2003 Revision of Standard Certificates (cont.) – Format of panel • “Parent group" that oversaw the process • Three subgroups that individually focused on content of birth, death, and fetal death certificates • A fourth subgroup for standards and design – Focused on formatting all the certificates and worksheets – Standardized record content to facilitate data comparability and compatibility – Made recommendations for implementation Vital Statistics Registrar Ref Manual 136 US STANDARD CERTIFICATES AND REPORTS 2003 Revision of Standard Certificates (cont.) – Three criteria guided the panel's decision in determining whether to keep an existing item or add a new item • Is the item needed for legal, research, statistical, or public health programs? • Is the item collectible with reasonable completeness and accuracy? • Is the vital statistics system the best source for this information? Vital Statistics Registrar Ref Manual 137 US STANDARD CERTIFICATES AND REPORTS 2003 Standard Certificate of Live Birth – Substantial changes particularly in medical portion • Revisions to medical risk factors, obstetric procedures, complications of labor and/or delivery, method of delivery, abnormal conditions of the newborn, congenital anomalies • Questions added about maternal morbidity, mother’s height and weight, WIC participation, principle method of payment for delivery, infections present, breast-feeding status – Checkbox items were re-designed to elicit more specific responses from data providers – Specific items were added to certificate to address data collection needs and to facilitate the linkage of data sets Vital Statistics Registrar Ref Manual 138 US STANDARD CERTIFICATES AND REPORTS 2003 Standard Certificate of Live Birth (cont.) – Reformatted to add section for “administrative use” • Contains items needed for statutory mandates other than those related to establishing permanent legal record such as mother’s mailing address, marital status, SSN requested for child, mother’s and father's SSNs, etc. – Other areas • Separate standardized worksheets were developed for the mother and for facility staff – Items to be completed by the mother were separated from medical information completed by facility staff – Worksheets included clear, unambiguous questions, definitions, instructions, and preferred data sources • The birth subgroup highly recommended testing the certificate and worksheets before final release to the states Vital Statistics Registrar Ref Manual 139 US STANDARD CERTIFICATES AND REPORTS 2003 Standard Report of Fetal Death – Applicable changes from the birth certificate were integrated into the fetal death report – Changes to cause of fetal death section • Changed to a checkbox format to improve quality of reporting • Revised and expanded to include additional medical items about the fetus – Worksheets about the patient and the delivery were developed for completion by facility staff – The fetal death subgroup also recommended that worksheets be tested prior to implementation Vital Statistics Registrar Ref Manual 140 US STANDARD CERTIFICATES AND REPORTS 2003 Standard Certificate of Death – Items were added to facilitate ICD-10 coding and to improve the quality of cause of death data • Did tobacco use contribute to death? • More information on pregnancy status at death for females • Decedent’s role if transportation accident – To improve reporting on sensitive items such as Occupation, business/industry, Hispanic origin, race and education of decedent, a section was designated "for statistical use only" – Extensive instructions for the medical certifier were added as a detachable page to the certificate – Instructions for the funeral director were also added as a separate page to the certificate Vital Statistics Registrar Ref Manual 141 US STANDARD CERTIFICATES AND REPORTS Implementation of 2003 Standard Certificates – 2003 standard birth certificate • Extensive changes to the items and format • Most states were using automated systems to collect birth information from medical facilities – 2003 changes meant major revisions were needed to computer programs – Many states already planned to move to an Internet-based electronic birth record system for birth data – Changing computer systems was extremely expensive and many states did not have funds available – NCHS was unable to provide any funding to assist in implementing the new certificates – Most states wanted to wait to implement 2003 format until funds were available to reengineer entire birth system Vital Statistics Registrar Ref Manual 142 US STANDARD CERTIFICATES AND REPORTS Implementation of 2003 Standard Certificates (cont.) – 2003 standard birth certificate (cont.) • As states reengineered their computer programs for an Internet-based electronic birth record system they used the 2003 birth certificate format – The timing was different for each state – Some states became “early adopters” of the 2003 certificate while others are still in the process of implementing the 2003 version – NCHS received different VSCP data sets from states causing processing and analysis problems Vital Statistics Registrar Ref Manual 143 US STANDARD CERTIFICATES AND REPORTS Implementation of 2003 Standard Certificates (cont.) – 2003 standard death certificate • Same problems with implementation applied to 2003 death certificate • Many states were developing Internet-based electronic death registration systems and decided to implement 2003 revision during this development • Again, NCHS did not have funding to support implementation • Some funding was available from the Social Security Administration because they wanted to improve timeliness of reporting the fact of death • As with births, states are at different stages in the implementation process for the 2003 death format with some states yet to implement Vital Statistics Registrar Ref Manual 144 US STANDARD CERTIFICATES AND REPORTS Implementation of 2003 Standard Certificates (cont.) – Problems with use of different versions • Different timing for state implementation meant different formats in states • NCHS had to handle two different formats for each certificate • Items on certificates were not always comparable from each revision • Data on new items was not available from all states for statistical analysis – To meet VSCP contract requirements, all states will be required to use 2003 standard certificate items formats beginning with 2014 data Vital Statistics Registrar Ref Manual 145 Section 9 Resources for 2003 Standard Certificates Vital Statistics Registrar Ref Manual 146 RESOURCES FOR 2003 STANDARD CERTIFICATES Worksheets for 2003 Standard Birth Certificate and Fetal Death Report – Recommended by panel reviewing the standard certificates for 2003 – Improve data quality and obtain more consistent information – Contain detailed instructions for completion of items – Indicate preferred data sources – Review panel suggested that states consider integrating fetal death reporting into their electronic birth registration system for ease of data entry by facility staff Vital Statistics Registrar Ref Manual 147 RESOURCES FOR 2003 STANDARD CERTIFICATES Worksheets for 2003 Standard Birth Certificate and Fetal Death Report (cont.) – Four standard worksheets • Mother's Worksheet for Child's Birth Certificate – Designed with questions to be completed by mother – Obtains legal and administrative information on mother and father – Also asks mothers height, weight, smoking, WIC participation – All medical information collected on facility worksheet • Facility Worksheet for Live Birth Certificate – Intended to be completed by facility staff – Information should be obtained from prenatal care records, mother's medical records, and labor and delivery records – Worksheet contains definitions and suggested sources for obtaining the information Vital Statistics Registrar Ref Manual 148 RESOURCES FOR 2003 STANDARD CERTIFICATES Worksheets for 2003 Standard Birth Certificate and Fetal Death Report (cont.) – Four standard worksheets (cont.) • Patient's Worksheet for the Report of Fetal Death – Designed to be completed by patient or for someone in the facility to use in interviewing the patient – Similar to mother’s worksheet for birth but has slight rewording • Facility Worksheet for the Report of Fetal Death – Similar to facility worksheet for live birth – Additional questions on cause of fetal death Vital Statistics Registrar Ref Manual 149 RESOURCES FOR 2003 STANDARD CERTIFICATES Worksheets for 2003 Standard Birth Certificate and Fetal Death Report (cont.) – Guide to Completing Facility Worksheet for the Certificate of Live Birth and Report of Fetal Death • Very detailed document developed to assist facility staff in completing the worksheets • Goes item by item in the order they appear on the worksheet – Definitions – Specific instructions for completing each item – Identifies source in medical record where information for each item may be found – Identifies alternative or synonymous terms and common abbreviations for the item Vital Statistics Registrar Ref Manual 150 RESOURCES FOR 2003 STANDARD CERTIFICATES Standard Specifications for Collecting and Editing Birth and Death Certificates – Background • In the late 1980s and early 1990s many states were beginning to automate collection of vital record information • By 2000 approximately 95% of births were registered electronically • Systems were developed in a piecemeal fashion in an era of constantly changing technology • States often depended on vendors who had limited specifications for their software • Significant data quality issues began to appear Vital Statistics Registrar Ref Manual 151 RESOURCES FOR 2003 STANDARD CERTIFICATES Standard Specifications for Collecting and Editing Birth and Death Certificates (cont.) – Panel reviewing standard certificates for 2003 recommended that NCHS develop and promulgate standards for vital statistics data collection and processing • Improve data quality and eliminate some of the problems in previous systems • Ensure uniformity in national data collection • Promote standardization and comparability among states • Consistent set of software specifications for use by all vendors and state offices Vital Statistics Registrar Ref Manual 152 RESOURCES FOR 2003 STANDARD CERTIFICATES Standard Specifications for Collecting and Editing Birth and Death Certificates (cont.) – Specifications included comprehensive set of instructions covering all aspects of the electronic system • Mechanisms for incorporating recommended worksheets into the system • Item specific edit criteria • Computational algorithms • Item code specifications • Response categories, including drop-down menus and “pick lists" • Requirement for context specific help • Electronic transmission standards Vital Statistics Registrar Ref Manual 153 RESOURCES FOR 2003 STANDARD CERTIFICATES Standard Specifications for Collecting and Editing Birth and Death Certificates (cont.) – Specific features recommended for inclusion in electronic software systems • Automatic edits at the time of data entry - allows users to immediately modify data • Ability to edit related items together - indicates problems with related items that are inconsistent • On-screen messages - item specific reminders/instructions • Online help - obtain more detailed instructions and definitions • Item order workflow - software entry should flow in same order as worksheets Vital Statistics Registrar Ref Manual 154 RESOURCES FOR 2003 STANDARD CERTIFICATES Standard Specifications for Collecting and Editing Birth and Death Certificates (cont.) – Specific features recommended for inclusion in electronic software systems (cont.) • Final review/query screen - allows user to temporarily skip items to gather information, and final screen reminds user to enter all missing information before records can be registered • List of pending items - allows user to easily access a list of incomplete items • Version control - changes in software versions should be tracked Vital Statistics Registrar Ref Manual 155 RESOURCES FOR 2003 STANDARD CERTIFICATES Standard Specifications for Collecting and Editing Birth and Death Certificates (cont.) – Additional information • Specifications were designed to be used with different types of electronic systems • Specifications follow as closely as possible data standards promulgated by CDC • Software meeting specifications should reduce the need for querying from states to providers and from NCHS to states • Prior to state implementation, NCHS evaluates a state's software to ensure it functions as intended and meets specifications Vital Statistics Registrar Ref Manual 156 RESOURCES FOR 2003 STANDARD CERTIFICATES Handbooks for 2003 Standard Death Certificate – Handbooks developed by NCHS • Previous versions updated for 2003 standard death certificate • Follows items as numbered on US Standard Death Certificate • Can be used as models for states to adapt for use in their own area – “Funeral Directors’ Handbook on Death Registration and Fetal Death Reporting” • General Information about death certificates and funeral director responsibilities • Item by item instructions Vital Statistics Registrar Ref Manual 157 RESOURCES FOR 2003 STANDARD CERTIFICATES Handbooks for 2003 Standard Death Certificate – “Physicians’ Handbook on Medical Certification of Death” • Emphasis on medical certification of death • Detailed examples on entering cause of death information – “Medical Examiners’ and Coroners’ Handbook on Death Registration and Fetal Death Reporting” • General information about death certificates and medical examiner or coroner responsibilities • Emphasis on medical certification of death with detailed examples Vital Statistics Registrar Ref Manual 158 Section 10 Vital Record Topics Vital Statistics Registrar Ref Manual 159 VITAL RECORD TOPICS Registration Issues – Establish legal documents required by law – Goal to register all vital events occurring in a state as they occur – Timeliness • Vital events should be registered within time period specified in law • Use of Electronic Birth Registration (EBRs) and Electronic Death Registration (EDRs) software has improved timeliness in most states • Some states still have problems meeting timeliness requirements of VSCP and SSA contracts Vital Statistics Registrar Ref Manual 160 VITAL RECORD TOPICS Registration Issues (cont.) – Timeliness (cont.) • Improving timeliness – – – – – – – – Consider eliminating requirements for paper records Accept electronic records before paper records are received Have electronic records filed directly with state office Do not delay birth registration if paternity documents have not been received within a short time (1-2 weeks) after birth Conduct quality checks on statistical items after record has been filed and sent to NCHS - send updated records later Monitor infant deaths throughout year and follow-up on any un-filed records Allow death records to be filed with cause of death “Pending” Give reports to hospitals and funeral directors showing how they compare with similar providers in submitting records Vital Statistics Registrar Ref Manual 161 VITAL RECORD TOPICS Registration Issues (cont.) – Quality • Edits should be built into EBR and EDR programs for data collection • EBRs and EDRs should have extensive “help” incorporated into the software • Additional edits should be done at state office for consistency or unusual trends • Check for duplicate records • Provide training to providers responsible for filing vital records – Classes and one-on-one training as needed – Detailed instruction manuals – Add a “help line” for providers to call Vital Statistics Registrar Ref Manual 162 VITAL RECORD TOPICS Registration Issues (cont.) – Quality (cont.) • Follow-up with provider to resolve problems • Monitor providers for high numbers of unknowns or other problems and provide additional training • Follow-up on all births with birth weight less than 750 grams to determine if infant died and if so, that a death record was filed • Query death records with incomplete cause of death information • Provide training to medical certifiers with high numbers of illdefined causes or incomplete entry of cause of death information Vital Statistics Registrar Ref Manual 163 VITAL RECORD TOPICS Registration Issues (cont.) – Field program • Furnishes outreach and support to providers and others involved in the vital statistics process • Traditional field program – Staff traveled around state to visit vital statistics providers and local registrars » » » » General contact to meet providers and local staff Answer questions Follow-up on problems with records Conduct training as needed – Full-time job of one or more staff members – Expensive and time consuming – Program varied from state to state as vital statistics funding and staffing became problematic Vital Statistics Registrar Ref Manual 164 VITAL RECORD TOPICS Registration Issues (cont.) – Field program (cont.) • Improvements in technology changed field program in many states – – – – – Allow contact with providers to be made more efficiently Follow-up with providers through phone calls, e-mail, and fax Provide training material on Internet Use social media to promulgate information “Help” provided within electronic data collection software • Examples of current staff field visits in many states – – – – To conduct training classes for new software implementation To specific providers for special training or to handle problems To hospitals to conduct audits of data provided on birth records To attend meetings of provider organizations Vital Statistics Registrar Ref Manual 165 VITAL RECORD TOPICS Birth/Fetal Death Registration – All live births should be registered • Does not depend on gestational age • Does not matter if infant alive or dead at time of registration • If infant born alive then dies – live birth should be registered – death should be registered – Fetal deaths should be registered as specified in state law – Infant deaths should not be reported as fetal deaths – For multiple pregnancies • Each member born alive is registered separately as a live birth • Members not born alive are registered as fetal deaths Vital Statistics Registrar Ref Manual 166 VITAL RECORD TOPICS Out of Hospital Births – Should be reported by • Medical facility where mother & child were examined within five days of birth • Physician or other licensed health provider who examined mother & child within five days of birth • Mother or father – Evidence required if birth registered within one year of birth • Evidence of pregnancy – Prenatal record – Statement from physician or health provider – Home visit by public health nurse or other health care provider • Evidence infant was born alive – Statement from healthcare provider who saw or examined infant – Observation of infant by public health nurse Vital Statistics Registrar Ref Manual 167 VITAL RECORD TOPICS Out of Hospital Births (cont.) – Evidence required (cont.) • Evidence of mother's presence in state on date of birth – If birth occurred at mother's residence » Rent receipt or utility bill with mother's name and address » State issued license with mother's current residence – If birth occurred outside of mother's place of residence and mother is a resident of state » Affidavit from tenant of premises where birth occurred stating mother was present on those premises at time of birth » Evidence of affiant's residence » Evidence of mother's residence in state – If mother not a resident of state, evidence that mother was in state at time of birth must be clear and convincing to state registrar Vital Statistics Registrar Ref Manual 168 VITAL RECORD TOPICS Delayed Births – Registered after time period specified in law • Births registered within one year of birth but after time period specified in law – Are considered delayed but not marked as delayed – Registered in standard format • Births registered more than one year after date of birth – Generally in special format – Marked delayed – Delayed registration of birth form should contain a description of each document submitted to support facts – Date of registration should be shown on certifications issued for a delayed birth along with a description of evidence used to file the record Vital Statistics Registrar Ref Manual 169 VITAL RECORD TOPICS Delayed Births (cont.) – Model law recommends all delayed records be filed at state office – Delayed reports of birth are not registered for deceased individuals – Documentary evidence is required to establish the following • • • • Full name of person at the time of live birth Date of live birth Place of live birth Full name of mother prior to first marriage Vital Statistics Registrar Ref Manual 170 VITAL RECORD TOPICS Delayed Births (cont.) – Documentary evidence • Must have been created at least one year prior to application for delayed birth • Must be from independent sources and in form of an original or duly certified copy of document • Generally at least three pieces are required • Evidence may vary for persons born before 1965 and those born on or after 1965 – Individuals not having sufficient documentary evidence are directed to seek a court order establishing the facts of birth – See Model Law and Regulations and NAPHSIS security guidelines for additional details on requirements Vital Statistics Registrar Ref Manual 171 VITAL RECORD TOPICS Amendments – Usually a change to a certification item after a certification of record has been issued • Provided for in state law and regulations • Documentary evidence needed for most amendments – Support alleged facts – Some corrections may require court determinations of facts – Procedure for amending record • Depends on type of record, error made, age of record • Notation should be kept with record showing documentary evidence used • Date amendment made and clerk making amendment should be noted • Original information should be preserved for audit purposes Vital Statistics Registrar Ref Manual 172 VITAL RECORD TOPICS Amendments (cont.) – Certification of amended record • • • • Should note that record was amended Should show item amended Should show date amendment was made May show original information in some cases – Special circumstances • Names on birth records may be amended in many states as follows – If a person obtains a legal name change through a court action, new name may be shown on birth record – Parents may request changes to the first and middle names of a child until the child's first birthday Vital Statistics Registrar Ref Manual 173 VITAL RECORD TOPICS Amendments (cont.) – Special circumstances (cont.) • Delayed records of birth may not be amended – Documentary evidence was used to place the record on file – Exception for certain legal actions • Medical certification of cause of death – Amended only upon request of the medical certifier who originally certified cause of death – Exceptions may be made in the absence of that certifier • Marital status on death records may require court determination depending on information originally shown on the record – If applicant has insufficient documentation to make requested amendment, applicant may appeal to a court of competent jurisdiction Vital Statistics Registrar Ref Manual 174 VITAL RECORD TOPICS Amendments (cont.) – Correction to records • Change to a non-certification item or in some cases to a certification item if no certifications have been issued • Record not marked amended • Used for – Minor errors – Typographical errors – Administrative errors – Obvious clerical errors • Made at discretion of state registrar • May not need documentation • Should be noted on the record in such a way as not to be shown in the certification Vital Statistics Registrar Ref Manual 175 VITAL RECORD TOPICS Replacement Birth Record after Adoption – Reports of adoption are received from courts • If person born in state, a replacement birth record is prepared containing information in court report of adoption – Court must provide sufficient information to identify birth record of adopted child – Replacement record of birth substituted for original record of birth – Original record of birth and documents submitted are placed under seal – Court may request that no replacement birth record be prepared – If adoption annulled by court, original record of birth is restored • If person born in another state, adoption report is forwarded to state of birth Vital Statistics Registrar Ref Manual 176 VITAL RECORD TOPICS Replacement Birth Record after Adoption (cont.) – Foreign born children adopted in state • Child not citizen of US at time of birth – Most states have provisions in their law for creating some type of birth document – Court order should show actual date and place (country) of child's birth – Birth document prepared by state » Should show country of child's birth » Should contain a statement indicating “Record of Foreign Live Birth" • Child is citizen of US at time of birth – Consular Report of Birth may be obtained from US Department of State Vital Statistics Registrar Ref Manual 177 VITAL RECORD TOPICS Replacement Birth Record after Parentage Determination – Replacement record of birth may be prepared in same manner as adoption – Method for determining parentage • Court determination of parentage • Establishment of parentage – Parents marry after birth of child » Sworn acknowledgment of paternity signed by both parents » Certification of parent’s marriage record – Unmarried parents » Acknowledgment of paternity signed by both biological parents » Written request signed by both parents to change child’s surname – If another man is shown as father of child on original record, court determination of paternity is needed prior to preparation of replacement birth record Vital Statistics Registrar Ref Manual 178 VITAL RECORD TOPICS Linking Birth and Death Records – Necessary to prevent fraudulent use of birth records – Match death records to live birth records • In state deaths • Out-of-state deaths received through IJE • Military deaths and deaths in foreign countries – Mark birth record as deceased – Document date of death and state or country of death with birth record – Certifications of birth records marked deceased should be similarly marked Vital Statistics Registrar Ref Manual 179 VITAL RECORD TOPICS Confidentiality of Vital Records Information – Necessary to • Protect individual privacy • Safeguard personal and medical information of individuals • Prevent fraud and identity theft – Should be specified in vital records law and regulations • Data or information contained in vital records should not be disclosed except as specified in law • Vital records should not be available for public inspection • Only authorized individuals should be able to obtain certifications or information from vital records Vital Statistics Registrar Ref Manual 180 VITAL RECORD TOPICS Confidentiality of Vital Records Information (cont.) – Care should be taken to prevent inadvertent release of personally identifiable information • Information that can be used to distinguish or trace an individual’s identity • Includes names, Social Security numbers, address • When linked to other personal or identifying information, such things as date and place of birth, medical information, facility where event occurred, etc. may be used to identify individuals – Disclosure of identifiable information for health and scientific research purposes • Personally identifiable information may generally be disclosed • Researcher must submit a written request • State registrar must approve request Vital Statistics Registrar Ref Manual 181 VITAL RECORD TOPICS Confidentiality of Vital Records Information (cont.) – Disclosure of identifiable information for health and scientific research purposes (cont.) • Researcher should sign written agreement that includes – – – – – – Details specifying name, title and organization of researcher Objectives and title of research study Intended uses of information obtained If study has institutional review board approval If contact will be made to any study subjects or next of kin Method for protecting the confidentiality and security of information provided – Provision for destruction of information at conclusion of study – Timeframe for study Vital Statistics Registrar Ref Manual 182 VITAL RECORD TOPICS Confidentiality of Vital Records Information (cont.) – Disclosure of identifiable information for health and scientific research purposes (cont.) • Researcher should sign written agreement that includes (cont.) – Names of persons on research team who will have access to confidential information – Plan for dissemination of study results – Prohibition of re-release by researcher of any personally identifiable information without explicit permission from state registrar – Acknowledgement by researcher that ownership of any vital record information provided remains with state registrar Vital Statistics Registrar Ref Manual 183 VITAL RECORD TOPICS Confidentiality of Vital Records Information (cont.) – Disclosure of identifiable information to government agencies in the conduct of their official duties • Should have written agreement signed by agency official • Agency should specify intended uses of information obtained from vital records • Confidentiality and security of information provided should be protected by agency • Agreement should prohibit re-release by of any personally identifiable information other than that spelled out in agreement or without explicit permission of the state registrar • Agency should acknowledge that ownership of any vital record information provided remains with state registrar Vital Statistics Registrar Ref Manual 184 VITAL RECORD TOPICS Confidentiality of Vital Records Information (cont.) – Programs/agencies commonly receiving files of identifiable information • For public health purposes – – – – – Immunization Newborn screening Newborn hearing Cancer registries Other disease or congenital anomalies registries • For administrative purposes (may be required by law) – – – – Voter registration Medicaid Motor vehicle licensing agency Law enforcement Vital Statistics Registrar Ref Manual 185 VITAL RECORD TOPICS Issuance of Certifications – One of the main functions of vital statistics office – Require application by qualified applicant • Should be signed, have identity documentation, evidence of eligibility • Qualified applicants – For birth records » » » » » » Registrant Registrant’s spouse (civil partner, domestic partner) Registrant’s child Registrant’s parent or legal guardian Legal or authorized representative Government agency in conduct of official duties Vital Statistics Registrar Ref Manual 186 VITAL RECORD TOPICS Issuance of Certifications (cont.) – Require application by qualified applicant (cont.) • Qualified applicants (cont.) – For death records » » » » » » » » Decedent’s spouse (civil partner, domestic partner) Decedent’s child Next of kin Decedent’s parent or legal guardian Legal or authorized representative Government agency in conduct of official duties Funeral director named on death record for 12 months after death Others who demonstrate that record is needed for determination or protection of applicant's personal or property right Vital Statistics Registrar Ref Manual 187 VITAL RECORD TOPICS Issuance of Certifications (cont.) – Certification format • Should be issued on security paper with appropriate features (see information on NAPHSIS website) • Notations on record such as “Deceased” or “Amended” should be shown • Information identified as being collected for health or medical purposes should not be shown • Should contain date of registration (previously called “date filed”) • Should contain date of issuance and statement of state registrar • Request number or tracking number should be shown • Issuing office (state or county/local) should be identified Vital Statistics Registrar Ref Manual 188 VITAL RECORD TOPICS Issuance of Certifications (cont.) – Preferably all certifications should be issued from centralized database • Remote sites should have access to centralized database with appropriate security restrictions • Information on applicants for certifications should be kept in a centralized database for tracking requests and security reasons • Centralized database should contain notation if there is an alert on record - registrant deceased on birth, record used for fraud, missing child, etc. • Software should have fee accounting functions with appropriate controls for supervisory staff to monitor employee actions • Automated reports with system activity and accounting data should be produced daily, weekly, monthly, and annually Vital Statistics Registrar Ref Manual 189 VITAL RECORD TOPICS Issuance of Certifications (cont.) – Certifications issued from centralized database (cont.) • Software should generate return information to customer – For appropriate requests - certification along with information letter and/or receipt – For inappropriate requests - information letter on problem with request or reason requester is not authorized to obtain record • Software should route request to appropriate unit for action certification, amendment, delayed birth, adoption, or parentage determination • System should track number of copies issued and date issued along with request or tracking number • Database of request information should be maintained and searchable for customer queries Vital Statistics Registrar Ref Manual 190 VITAL RECORD TOPICS Issuance of Certifications (cont.) – Certifications issued from centralized database (cont.) • Automated systems should have appropriate security features – System should be designed so electronic files cannot be tampered with – System should permanently record all changes made - who what where when etc. – Password, log on, biometric identifier, etc. should be required for persons to enter system – Authorization for employees to conduct functions in system should be assigned by a supervisor – System should be designed so that at least two people are necessary to process a request for certification - for example money handling separate from production of certification – A log trail should exist for any actions taken against any record in the system Vital Statistics Registrar Ref Manual 191 VITAL RECORD TOPICS Issuance of Certifications (cont.) – Certifications should be issued in a timely manner • Performance standards recommend – For walk-in requests, within 30 min. – For mail requests, 0 to 3 days – Problem requests should be responded to promptly • Explain problem to applicant • Provide information on way to fix problem • Request should be tracked in system for future reference and/or queries Vital Statistics Registrar Ref Manual 192 Section 11 Statistical Data from Vital Records Vital Statistics Registrar Ref Manual 193 STATISTICAL DATA FROM VITAL RECORDS General Principles for Vital Statistics – Complete, accurate, timely data from all vital events – Must meet needs of statistical users • Include sufficient detail • Be relevant – Data items must have clear, explicit definitions • Follow national standards • Be comparable over time and to other data systems – Statistical methods should be documented and made available to users – Limitations of the data should be specified Vital Statistics Registrar Ref Manual 194 STATISTICAL DATA FROM VITAL RECORDS General Principles for Vital Statistics (cont.) – Classifications and groupings for tabulations should follow recognized standards – Tabulations should have continuity over time – Data should be readily accessible – Procedures should be in place to prevent inadvertent release of personally identifiable information – System must be able to adapt to new and changing technology Vital Statistics Registrar Ref Manual 195 STATISTICAL DATA FROM VITAL RECORDS Uses of Vital Statistics – – – – – – – – – Public health and epidemiologic monitoring Study trends in mortality and natality Investigate the prevalence and distribution of diseases Identify populations at risk for certain medical problems and diseases Examine differences among population groups Construct life tables Establish baseline levels Evaluate effectiveness of public health programs Study maternal and child health and infant mortality Vital Statistics Registrar Ref Manual 196 STATISTICAL DATA FROM VITAL RECORDS Uses of Vital Statistics (cont.) – – – – – – – – – Help identify local health problems and issues Conduct demographic studies Prepare population estimates and projections Planning and allocation of resources by government agencies Consumer market research Determining retail and service locations Forecasting future trends Planning for business growth Studying economic and social conditions Vital Statistics Registrar Ref Manual 197 STATISTICAL DATA FROM VITAL RECORDS Presentation of Vital Statistics Data – Tabulations • Display numerical data • Allow users to make comparisons and note relationships between data sets • Degree of detail depends on purpose of table – Standard groupings should be used for comparisons with national and other states’ data – Special groupings may be used to meet user needs or study a particular problem • Time period used should be specified • Usually data are tabulated by place of residence, but place of occurrence may be used for some purposes Vital Statistics Registrar Ref Manual 198 STATISTICAL DATA FROM VITAL RECORDS Presentation of Vital Statistics Data (cont.) – Charts, graphs and figures • Allow users to more easily determine meaning of numbers • Patterns of data stand out • Used to show – Distributions of data – Patterns over time – Relationships at a point in time • Consider use of chart and level of user – Statistical sophistication of user – Complexity of data shown Vital Statistics Registrar Ref Manual 199 STATISTICAL DATA FROM VITAL RECORDS Examples of Natality Statistics – Tabulations showing • • • • Live birth numbers and rates with time trends Detail about mother - age, race or ethnicity, marital status Detail about infant - sex, birth weight, congenital malformations Pregnancy information - prenatal care, birth order, attendant at birth, period of gestation, medical problems of mother • Geographic information - appropriate small area information by place of residence of mother • Detail for teenage births - age, race or ethnicity, prenatal care, birth weight, residence Vital Statistics Registrar Ref Manual 200 STATISTICAL DATA FROM VITAL RECORDS Examples of Natality Statistics (cont.) – Natality calculations such as • • • • • • • • Crude birth rates by geographic region Percent of births by age of mother Age specific birth rates Percent of births by birth weight Percent of preterm births Percent of births receiving prenatal care in the first trimester Fertility rates Adequacy of prenatal care (For detail on calculations see NCHS publications and/or NAPHSIS website) Vital Statistics Registrar Ref Manual 201 STATISTICAL DATA FROM VITAL RECORDS Examples of Mortality Statistics – Tabulations showing • Death numbers and rates with time trends • Demographic information about deceased - age, sex, race or ethnicity, marital status • Geographic information - appropriate small area information by place of residence of deceased • Cause of death information - detail on leading causes of death by age, sex, and place of residence of deceased • Detail on infant mortality, perinatal mortality, and maternal mortality Vital Statistics Registrar Ref Manual 202 STATISTICAL DATA FROM VITAL RECORDS Examples of Mortality Statistics (cont.) – Mortality calculations such as • • • • • • • • • Crude death rate by geographic region Percent of deaths by age of decedent Age specific death rates Age-adjusted death rates Cause specific death rates Infant mortality rates Maternal mortality rates Life expectancy at birth Years of potential life lost (For detail on calculations see NCHS publications and/or NAPHSIS website) Vital Statistics Registrar Ref Manual 203 STATISTICAL DATA FROM VITAL RECORDS Dissemination of Vital Statistics – Should be timely • Release as quickly as possible - don’t hold for printed material • Use Internet - include as many publications as possible – Should use multiple formats • Annual reports – Detail for reference – Historical • Preliminary data publications • News releases • Ad hoc tabulations Vital Statistics Registrar Ref Manual 204 STATISTICAL DATA FROM VITAL RECORDS Dissemination of Vital Statistics (cont.) – Should use multiple formats (cont.) • Special summaries on particular topics – – – – – Maternal and child health Infant deaths Particular causes of death Local area (county/city) data Annual statistical highlights • Public use data files - may not be feasible in small states • Interactive web-based data query systems – Allow users to create their own tables and graphs – Should contain limited or grouped data to protect privacy Vital Statistics Registrar Ref Manual 205 STATISTICAL DATA FROM VITAL RECORDS Dissemination of Vital Statistics (cont.) – Include technical information • • • • • • • • • Methods of data collection Definitions of items Processing procedures Data quality assessments Limitations on data usage Formulas used and other details for calculations Imputation methods ICD code groupings used Population data for denominators Vital Statistics Registrar Ref Manual 206 STATISTICAL DATA FROM VITAL RECORDS Special Use Files – For statistical or health research • May be provided if state does not produce public use files or they do not meet needs of user • Some states allow researchers to have access to files on agency premises under staff supervision • State statisticians may provide analytic consultation – May produce tabulations or link files – May co-author research papers • May charge for creation of files or consultation • Users must submit research request and sign confidentiality agreement Vital Statistics Registrar Ref Manual 207 STATISTICAL DATA FROM VITAL RECORDS Special Use Files (cont.) – Linked files for statistical or health research • Researchers must sign confidentiality agreement – Should state that linked data will remain confidential – Should place limitations on use of linked file • Examples of linked files – Deaths linked to births » Infant death linked to birth » Maternal death linked to birth or fetal death – Births linked to Medicaid files – Births linked to government social or benefit programs » Births to WIC (Women, Infants, and Children) recipients » Births to TANF (Temporary Assistance for Needy Families) recipients – Deaths to Medicaid (or Medicare) files – Deaths to census records Vital Statistics Registrar Ref Manual 208 Section 12 Cause of Death Tabulation Vital Statistics Registrar Ref Manual 209 CAUSE OF DEATH TABULATION Collection of Cause of Death Information on Death Certificates – Medical Certification of Death form recommended by World Health Organization (WHO) – US has added additional fields • To obtain more detailed information related to injuries • To obtain more detail for coding cause of death Cause of Death Information Completed by Medical Certifier – Physician in attendance – Coroner or medical examiner in some circumstances Vital Statistics Registrar Ref Manual 210 CAUSE OF DEATH TABULATION All Causes of Death Should Be Entered on Medical Certification – WHO says causes to be entered are "all those diseases, morbid conditions or injuries which either resulted in or contributed to death and the circumstances of the accident or violence which produced any such injuries" – Underlying cause of death • Defined by WHO as "(a) the disease or injury which initiated the train of morbid events leading directly to death, or (b) the circumstances of the accident or violence which produced the fatal injury" • Out of all causes listed on death certificate, the cause that initiates the chain of events leading to death • Used for tabulation purposes • Most useful statistic for public health analysis Vital Statistics Registrar Ref Manual 211 CAUSE OF DEATH TABULATION Defined Sequence for Entering Causes of Death – Part I of form should contain • Diseases in chain of events leading to death • Condition leading directly to death on first line • Each step in chain of events leading to direct cause of death on following lines • Underlying cause starting chain of events leading to death on lowest line • WHO definition indicates symptoms or modes of dying such as respiratory arrest or heart failure should not be entered – Part II of form should contain • Other significant conditions contributing to death but not directly related to the death Vital Statistics Registrar Ref Manual 212 CAUSE OF DEATH TABULATION International Classification of Diseases (ICD) – – – – Maintained by World Health Organization (WHO) Used since late 19th century Since 1999, Tenth Revision (ICD-10) used in US Allows for comparisons of mortality data at various levels of geography including international, state, and local – Standards for classifying causes of death • • • • Codes and subgroups of codes Rules for applying codes and choosing underlying cause Lists for tabulating mortality statistics Standard definitions Vital Statistics Registrar Ref Manual 213 CAUSE OF DEATH TABULATION ICD-10 Consists of Three Volumes – Volume 1 contains the tabular list • Alphanumeric list of codes with associated category titles for diseases, injuries, external causes of injury, and factors related to health status in 22 chapters • ICD codes are three character categories (a letter and two numbers) with four character subcategories (the three characters followed by a decimal point and one number) • Possible range of codes is A00.0 to Z99.9 • Much more detailed than previous versions of ICD - about 8000 categories Vital Statistics Registrar Ref Manual 214 CAUSE OF DEATH TABULATION ICD-10 Consists of Three Volumes (cont.) – Volume 2 • Contains background on the ICD • Gives instructions on coding causes of death in selecting the underlying cause • Includes information on presentation of coded statistical data and calculation of statistical indicators – Volume 3 • Alphabetical index to classifications with their codes Vital Statistics Registrar Ref Manual 215 CAUSE OF DEATH TABULATION Applying ICD Codes to Cause of Death – Medical certifier should enter causes of death so that cause on lowest line in part one is underlying cause of death for statistical tabulations – Medical certifier may not enter cause correctly – Rules in ICD-10 Volume 2 used for selecting underlying cause of death • Applying rules requires extensive training • Underlying cause should be determined by trained nosologist – US uses computer programs to apply rules for choosing underlying cause • Computer assigns ICD codes to each cause of death entry and applies rules to select underlying cause • Computer program cannot always make selection • Some cases rejected for coding by trained nosologist Vital Statistics Registrar Ref Manual 216 CAUSE OF DEATH TABULATION Coding Underlying Cause of Death in US – Originally, states had trained nosologists – States sent coded data to NCHS – NCHS developed Mortality Medical Data System (MMDS) • Computer programs to automate entry and processing of cause of death data – SuperMICAR - allows literals to be entered – MICAR - automates multiple cause coding rules and assigns ICD codes to entries – ACME - applies WHO rules for selecting underlying cause of death – TRANSAX - translates output from ACME into better form for statistical tabulation Vital Statistics Registrar Ref Manual 217 CAUSE OF DEATH TABULATION Coding Underlying Cause of Death in US (cont.) – Mortality Medical Data System (MMDS) (cont.) • Rejected records still had to be coded by nosologists in state – NCHS began coding cause of death data for states without nosologists • States had difficulty maintaining trained staff • Some states began sending files of literals to be coded by NCHS • NCHS processed the data files and returned coded data and underlying cause codes back to the states – In 2011 NCHS decided to code cause of death data for all states Vital Statistics Registrar Ref Manual 218 CAUSE OF DEATH TABULATION Tabulation of Cause of Death Data – Tabulation lists were developed by WHO for presenting cause of death data • Lists give standard groups of 3-digit ICD-10 codes for presentation of underlying causes of death – For example: Malignant neoplasms is C00-C97; Diabetes mellitus is E10-E14; Accidents is V01-X59,Y85-Y86 – Standard groups of ICD codes are provided for general mortality data and for infant and child mortality data • US uses modified versions of lists developed by WHO • Lists are used for tabulation and distribution of mortality data by NCHS and states • Use provides consistency and comparability of mortality data between areas and over time Vital Statistics Registrar Ref Manual 219 CAUSE OF DEATH TABULATION Tabulation of Cause of Death Data (cont.) – Leading causes of death • Standard groups of ICD-10 codes from tabulation list are ranked • Specific list (113 Selected Causes of Death) used for choosing leading causes in US – Only certain groups from the list are eligible for ranking – Ill-defined conditions and residual groups are not used for ranking • Groups with largest number of deaths are leading causes Vital Statistics Registrar Ref Manual 220 CAUSE OF DEATH TABULATION Comparing Cause of Death Over Time – Deaths may be coded under different versions of the ICD (for example, ICD-9 versus ICD-10) • Coding rules and rules for selecting underlying cause of death may change • Trend discrepancies may be caused by different ICD versions • Rankings of leading causes of death may also be affected – Comparability studies • NCHS determines differences in ICD versions – A sample of death certificates are coded using both ICD versions – Underlying cause of death is determined using both versions Vital Statistics Registrar Ref Manual 221 CAUSE OF DEATH TABULATION Comparing Cause of Death Over Time (cont.) – Comparability studies (cont.) • Comparability ratio is developed – Number of deaths classified by new ICD divided by number of deaths classified by previous ICD – Can be used to adjust causes of death classified under previous ICD to be comparable to deaths classified under new ICD • From ICD-9 to ICD-10 some major changes to causes of death such as – – – – Septicemia Influenza and pneumonia Alzheimer’s disease Nephritis, nephrotic syndrome and nephrosis Vital Statistics Registrar Ref Manual 222