Ch. 1_Chemical Foundations PPT
advertisement
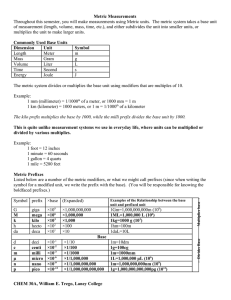
. Do Now: 1. Differentiate between qualitative and quantitative observations/data. 2. True or False (explain why): A theory can be proven correct or incorrect 1.1 Chemistry: An Overview Considered the “central” science ALL matter and energy transformations are based on interactions between atoms and molecules Macroscopic vs. submicroscopic views Science: a process for understanding the natural world and its changes 1.2 Scientific Method Making observations Qualitative Observations - observations made by using your senses (does not involve a number) Quantitative Observations - observations made by measuring (involves a number and a unit) Formulating hypotheses hypothesis - a possible explanation for an observation Performing experiments experiments - performed to test the hypothesis. Experiments will either support or disprove your hypothesis. Forming a theory - set of hypotheses that agree with observations over the test of time. Helps create a model. 1.3 Units of Measurement Metric System: ALL measurements in this course are expected to be reported in the metric system Measurements Number and Scale (units) are both essential "The number without the units is worthless!“ SI system Important SI Units for Chemistry Mass kilogram kg Length meter m Time second s Temperature Kelvin K Amount of Substance mole mol Volume liter L SI Prefixes Mega M 1,000,000 Kilo K 1,000 Hecto H 100 Deka D 10 Deci d .1 Centi c .01 Milli m .001 Nano n .000 000 001 Pico p .000 000 000 001 **KNOW TABLE 1.2 (page 9)** 106 103 102 100 10-1 10-2 10-3 10-9 10-12 1.4 Uncertainty in Measurement Every measurement always has some degree of uncertainty. When measuring it is always important to record the certain digits (those that are marked on the measuring device) and the first uncertain digit (not marked on the device and the number is guessed at). Accuracy - refers to the agreement of a particular value with the true value. Precision - refers to the degree of agreement among several measurements of the same quantity. Random error - means that a measurement has an equal probability of being high or low. Systematic error - this error occurs in the same direction each time; it is either always high or always low. 1.5 Significant Figures and Calculations Nonzero integers - nonzero integers always count as significant figures. Zeros - depending on the type of zero, it will determine weather the zero counts or does not. Leading zeros - are zeros that precede all the nonzero digits. these do not count as significant figures, they are considered place holders. Captive zeros - are zeros between nonzero digits. These always count as significant figures. Trailing zeros -are zeros at the right end of the number. They are significant only if the number contains a decimal point. Exact numbers - numbers that were not measured but determined by counting. These can be assumed to have an infinite number of significant figures. Mathematical Operations For multiplication or division - the number of significant figures in the result is the same as the number in the least precise measurement used in the calculation. For addition or subtraction - the result has the same number of decimal places as the least precise measurement used in the calculation. Rounding In a series of calculations, carry the extra digits through to the final result, then round. If the digit to be removed is less then 5, the preceding digit stays the same. is equal to or greater than 5, the preceding digit is increased by 1. 1.6 Dimensional Analysis method used to change from one system of units to another. Converting from one unit to another To convert from one unit to another, use the equivalence that relates the two units. Derive the appropriate unit factor (equivalence factor) by looking at the direction of the required change (to cancel the unwanted units) Multiply the quantity to be converted by the unit factor to give the quantity with the desired units. 1.7 Temperature Know the relationship and conversion between the three systems for measuring temperature Kelvin Celcius Farenheit (oF) (K) (oC) Celsius (°C) and Kelvin (K) Kelvin = Celsius + 273.15 Celsius = Kelvin - 273.15 Size of the temperature unit (degree) is the same Fahrenheit TC = (TF - 32°F)(5°C/9°F) TF = TC x (9°F/5°C) + 32°F 1.8 Density Derived unit Based on relationship between mass and volume D = M/V Dependent on temperature WHY? NOT dependent on amount or quantity of sample used 1.9 Classification of matter Matter Anything that occupies space and has mass States of Matter Solids - rigid, fixed volume and shape Liquids - definite volume, no specific shape Gases - no fixed volume or shape, highly compressible Mixtures - Matter of variable composition Heterogeneous mixtures Having visibly distinguishable parts Homogeneous mixtures (solutions) Having visibly indistinguishable parts Components of Mixtures can be Separated by Physical Means Distillation-difference in volatility Filtration-solid/liquid Chromatography—difference in polarity Pure substances Elements Cannot be decomposed into simpler substances by physical or chemical means Compounds Constant composition Can be broken into simpler substances by chemical means, some physical means HOMEWORK 1- Due Mon 9/10 A student performed an analysis of a sample for its Iodine content and got the following results. 19.3%, 21.2%, 18.7% and 20.4% The actual value is 20.3%. What can you say about the student’s accuracy and precision? What can you say about the cause(s) for any error? 2. Is the boiling of water a physical or chemical change? Explain 3. Textbook Questions 29a,b, 31a,b, 33a-d, 35c, 43, 45 1. Homework 2-Due Wed 9/12 Textbook Questions, Chapter 1 # 15, 47, 53, 59, 63, 67, 69, 77,