Fine and hyperfine structure of hydrogen
advertisement
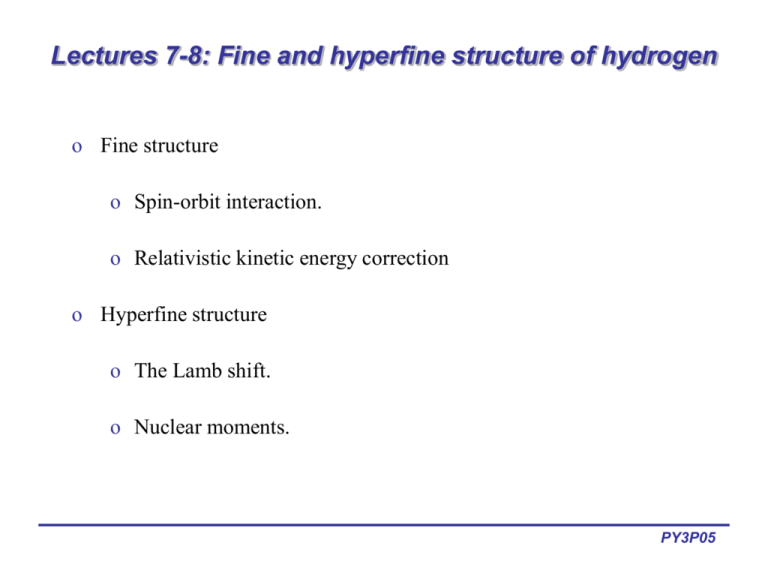
Lectures 7-8: Fine and hyperfine structure of hydrogen o Fine structure o Spin-orbit interaction. o Relativistic kinetic energy correction o Hyperfine structure o The Lamb shift. o Nuclear moments. PY3P05 Spin-orbit coupling in H-atom o Fine structure of H-atom is due to spin-orbit interaction: E so o Jˆ is a max Z Sˆ Lˆ 2 3 2m cr If L is parallel to S => J is a maximum => high energy configuration. Lˆ o +Ze 1 Sˆ Lˆ ( Jˆ Jˆ Lˆ Lˆ Sˆ Sˆ ) 2 Sˆ Lˆ 2 2 -e Angular momenta are described in terms of quantum numbers, s, l and j: Jˆ Lˆ Sˆ Jˆ 2 ( Lˆ Sˆ )( Lˆ Sˆ ) Lˆ Lˆ SˆSˆ 2 Sˆ Lˆ Sˆ Jˆ is a min Lˆ +Ze -e [ j( j 1) l(l 1) s(s 1)] Sˆ Z 3 1 E so [ j( j 1) l(l 1) s(s 1)] 4m 2c r 3 PY3P05 Spin-orbit effects in H fine structure o For practical purposes, convenient to express spin-orbit coupling as a j j 1 l(l 1) ss 1 2 where a Ze 20 2 /8m 2 r 3 is the spin-orbit coupling constant. Therefore, for the 2p electron: E a 3 3 11(1 1) 1 1 1 1 a so 2 2 2 2 2 2 E so a 1 1 1 1 E so 11(1 1) 1 a 2 2 2 2 2 E j = 3/2 +1/2a Angular momenta aligned j = 1/2 -a Angular momenta opposite 2p1 PY3P05 Spin-orbit coupling in H-atom o The spin-orbit coupling constant is directly measurable from the doublet structure of spectra. o If we use the radius rn of the nth Bohr radius as a rough approximation for r (from Lectures 1-2): n2 2 r 4 0 2 mZe Z4 a ~ 6 n o Spin-orbit coupling increases sharply with Z. Difficult for observed for H-atom, as Z = 1: 0.14 Å (H), 0.08 Å (H), 0.07 Å (H). o Evaluating the quantum mechanical form, o Therefore, using this and s = 1/2: Z4 a~ 3 n l(l 1)(2l 1) Z 2 4 2 [ j( j 1) l(l 1) 3/4] E so mc 2n 3 l(l 1)(2l 1) PY3P05 Term Symbols o Convenient to introduce shorthand notation to label energy levels that occurs in the LS coupling regime. o Each level is labeled by L, S and J: o o o o 2S+1L J L = 0 => S L = 1 => P L = 2 =>D L = 3 =>F o If S = 1/2, L =1 => J = 3/2 or 1/2. This gives rise to two energy levels or terms, 2P3/2 and 2P1/2 o 2S + 1 is the multiplicity. Indicates the degeneracy of the level due to spin. o If S = 0 => multiplicity is 1: singlet term. o If S = 1/2 => multiplicity is 2: doublet term. o If S = 1 => multiplicity is 3: triplet term. o Most useful when dealing with multi-electron atoms. PY3P05 Term diagram for H fine structure o The energy level diagram can also be drawn as a term diagram. o Each term is evaluated using: 2S+1LJ o For H, the levels of the 2P term arising from spin-orbit coupling are given below: 2P 3/2 E +1/2a Angular momenta aligned 2p1 (2P) 2P 1/2 -a Angular momenta opposite PY3P05 Hydrogen fine structure o Spectral lines of H found to be composed of closely spaced doublets. Splitting is due to interactions between electron spin S and the orbital angular momentum L => spin-orbit coupling. o H line is single line according to the Bohr or Schrödinger theory. Occurs at 656.47 nm for H and 656.29 nm for D (isotope shift, ~0.2 nm). o H Spin-orbit coupling produces fine-structure splitting of ~0.016 nm. Corresponds to an internal magnetic field on the electron of about 0.4 Tesla. PY3P05 Relativistic kinetic energy correction o According to special relativity, the kinetic energy of an electron of mass m and velocity v is: p2 p4 T 2m 8m 3c 2 o The first term is the standard non-relativistic expression for kinetic energy. The second term is the lowest-order relativistic correction to this energy. o Using perturbation theory, it can be show that E rel 1 Z 2 4 3 3 mc 2 2l 1 8n n o Produces an energy shift comparable to spin-orbit effect. o A complete relativistic treatment of the electron involves the solving the Dirac equation. PY3P05 Total fine structure correction o For H-atom, the spin-orbit and relativistic corrections are comparable in magnitude, but much smaller than the gross structure. Enlj = En + EFS o Gross structure determined by En from Schrödinger equation. The fine structure is determined by Z 4 4 3 2 1 EFS E so E rel mc 2l 1 8n 2n 3 o As En = -Z2E0/n2, where E0 = 1/22mc2, we can write o Z 2 E 0 Z 2 2 1 3 E H atom 2 1 n n j 1/2 4n Gives the energy of the gross and fine structure of the hydrogen atom. PY3P05 Fine structure of hydrogen o Energy correction only depends on j, which is of the order of 2 ~ 10-4 times smaller that the principle energy splitting. o All levels are shifted down from the Bohr energies. o For every n>1 and l, there are two states corresponding to j = l ± 1/2. o States with same n and j but different l, have the same energies (does not hold when Lamb shift is included). i.e., are degenerate. o Using incorrect assumptions, this fine structure was derived by Sommerfeld by modifying Bohr theory => right results, but wrong physics! PY3P05 Hyperfine structure: Lamb shift o Spectral lines give information on nucleus. Main effects are isotope shift and hyperfine structure. o According to Schrödinger and Dirac theory, states with same n and j but different l are degenerate. However, Lamb and Retherford showed in 1947 that 22S1/2 (n = 2, l = 0, j = 1/2) and 22P1/2 (n = 2, l = 1, j = 1/2) of H-atom are not degenerate. o Experiment proved that even states with the same total angular momentum J are energetically different. PY3P05 Hyperfine structure: Lamb shift 1. Excite H-atoms to 22S1/2 metastable state by e- bombardment. Forbidden to spontaneuosly decay to 12S1/2 optically. 2. Cause transitions to 22P1/2 state using tunable microwaves. Transitions only occur when microwaves tuned to transition frequency. These atoms then decay emitting Ly line. 3. Measure number of atoms in 22S1/2 state from H-atom collisions with tungsten (W) target. When excitation to 22P1/2, current drops. 4. Excited H atoms (22S1/2 metastable state) cause secondary electron emission and current from the target. Dexcited H atoms (12S1/2 ground state) do not. PY3P05 Hyperfine structure: Lamb shift o According to Dirac and Schrödinger theory, states with the same n and j quantum numbers but different l quantum numbers ought to be degenerate. Lamb and Retherford showed that 2 S1/2 (n=2, l=0, j=1/2) and 2P1/2 (n=2, l=1, j=1/2) states of hydrogen atom were not degenerate, but that the S state had slightly higher energy by E/h = 1057.864 MHz. o Effect is explained by the theory of quantum electrodynamics, in which the electromagnetic interaction itself is quantized. o For further info, see http://www.pha.jhu.edu/~rt19/hydro/node8.html PY3P05 Hyperfine structure: Nuclear moments o Hyperfine structure results from magnetic interaction between the electron’s total angular momentum (J) and the nuclear spin (I). o Angular momentum of electron creates a magnetic field at the nucleus which is proportional to J. o ˆ nucleus Bˆ electron Iˆ Jˆ Interaction energy is therefore E hyperfine o Magnitude is very small as nuclear dipole is ~2000 smaller than electron (~1/m). o Hyperfine splitting is about three orders of magnitude smaller than splitting due to fine structure. PY3P05 Hyperfine structure: Nuclear moments o o o o Like electron, the proton has a spin angular momentum and an associated intrinsic dipole moment e ˆ p gp Iˆ M The proton dipole moment is weaker than the electron dipole moment by M/m ~ 2000 and hence the effect is small. be shown to be: Resulting energy correction can gpe 2 ˆ ˆ E p IJ mMc 2 r 3 Total angular momentum including nuclear spin, orbital angular momentum and electron spin is Fˆ Iˆ Jˆ where F f ( f 1) Fz m f o The quantum number f has possible values f = j + 1/2, j - 1/2 since the proton has spin 1/2,. o Hence every energy level associated with a particular set of quantum numbers n, l, and j will be split into two levels of slightly different energy, depending on the relative orientation of the proton magnetic dipole with the electron state. PY3P05 Hyperfine structure: Nuclear moments o The energy splitting of the hyperfine interaction is given by a E HFS f f 1 i(i 1) j j 1 2 where a is the hyperfine structure constant. o o E.g., consider the ground state of H-atom. Nucleus consists of a single proton, so I = 1/2. The hydrogen ground state is the 1s 2S1/2 term, which has J = 1/2. Spin of the electron can be parallel (F = 1) or antiparallel (F = 0). Transitions between these levels occur at 21 cm (1420 MHz). For ground state of the hydrogen atom (n=1), the energy separation between the states of F = 1 and F = 0 is 5.9 x 10-6 eV. F=1 F=0 QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. 21 cm radio map of the Milky Way PY3P05 Selection rules o Selection rules determine the allowed transitions between terms. n = any integer l = ±1 j = 0, ±1 f = 0, ±1 PY3P05 Summary of Atomic Energy Scales o Gross structure: o Covers largest interactions within the atom: o Kinetic energy of electrons in their orbits. o Attractive electrostatic potential between positive nucleus and negative electrons o Repulsive electrostatic interaction between electrons in a multi-electron atom. o Size of these interactions gives energies in the 1-10 eV range and upwards. o Determine whether a photon is IR, visible, UV or X-ray. o Fine structure: o Spectral lines often come as multiplets. E.g., H line. => smaller interactions within atom, called spin-orbit interaction. o Electrons in orbit about nucleus give rise to magnetic moment of magnitude B, which electron spin interacts with. Produces small shift in energy. o Hyperfine structure: o Fine-structure lines are split into more multiplets. o Caused by interactions between electron spin and nucleus spin. o Nucleus produces a magnetic moment of magnitude ~B/2000 due to nuclear spin. o E.g., 21-cm line in radio astronomy. PY3P05