Unit Plan
advertisement

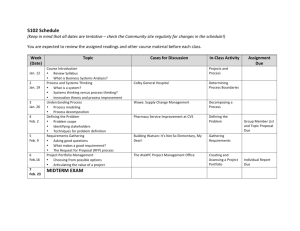
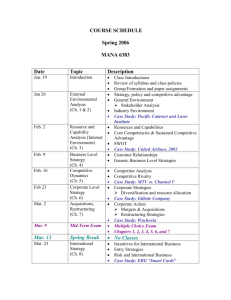
Unit A: Investigating Properties of Matter Science 14 Key: Content, Homework/Formative Assessment, Summative: Assignments, Unit Tests, Final Chapter 1 Day 1 -Introductions -Course Outline -Google Classroom Chapter 1 Day 2 -1.1 Chemicals All Around Us Notes -Lab Safety Notes Chapter 1 Day 3 -1.2 Describing Matter Notes Chapter 1 Day 4 -1.3 Classifying Matter Notes -1.2 Chemical & Physical Properties Notes -Classifying Matter Practice Q’s Chapter 1 Day 5 Chapter 1 Review Pg. 20 -WHMIS Notes Chapter 1 Day 6 -Check Your Understanding Pg. 9 #1-4 Chapter 2 -Check Your Understanding Pg. 15 #1-4 -2.1 Putting the Pieces Together Assignment 1 Due PD DAY Chapter 1 Test M (Feb 16) T (Feb 17) W (Feb 18) FAMILY DAY -2.2 Elements and Compounds -Chapter 2.1 & 2.2 Assignment NO CLASSES -Check Your Understanding Pg. 34 #1-3 -2.3 Chemical Names and Formulas M (Feb 23) T (Feb 24) W (Feb 25) PD DAY -Chapter 2 Test -3.1 Two Kinds of Mixtures NO CLASSES -Check Your Understanding Pg. 44 #1-3 -Solutions Practice Worksheet M (Mar 2) T(Mar 3) W(Mar 4) -Pg. 28 Fill in Chart -Check Your Understanding Pg. 30 #1, 2, 4 TH (Feb 19) NO CLASSES -Pg. 36 Fill in Chart -Check Your Understanding Pg. 37 #1-3 -Counting Atoms Worksheet TH (Feb 26) Chapter 2 Review Pg. 38 -3.2 What are Solubility and Concentration? -3.3 Separating Mixtures -How Does Temp Affect Sol? Pg. 47 Graphing -Check Your Understanding Pg. 50 #3-5 TH (Mar 5) F (Feb 20) F (Feb 27) -Check Your Understanding Pg. 55 #1-4 -Solubility Graphing Practice F(Mar 6) 1 -Solubility Graphing Assignment Chapter 3 Review -Chapter 3 Test Pg. 56 #1-12 NO CLASSES NO CLASSES M (Mar 9) T (Mar 10) W (Mar 11) TH (Mar 12) F (Mar 13) -4.1 Use Concentrated Solutions to Reduce Garbage -4.2 Acids and Bases -4.3 Acids and Bases in Action Chapter 4 Review Pg. 74 #1-17- Chapter 4 Quiz -Check your understanding pg. 69 -Check your understanding pg. 73 M (Mar 16) T (Mar 17) W (Mar 18) -Unit A Review Qs -Unit A Review Q’s Take Up UNIT A TEST -Check your understanding pg. 62 -Unit A Review TH (Mar 19) F (Mar 20) Science 14 Unit A: Investigating Properties of Matter UNIT OBJECTIVES Students will: 1. Classify various forms of matter, including commonly used household substances, on the basis of their properties, and relate these properties to their safe use, storage and disposal •describe the need for safety precautions that should be followed when handling, storing and disposing of substances at home and in the laboratory; and explain the WHMIS and consumer product symbols for labelling substances (e.g., flammable, corrosive, reactive, health hazard) •describe the importance of mixtures and solutions in household products (e.g., baking soda, soaps, paints) •compare and contrast the properties of pure substances and mixtures (e.g., brass and zinc, stainless steel and iron, acetic acid and vinegar, pure water and salt water), and relate this information to practical applications (e.g., salting icy roads, adding antifreeze to car radiators) •outline the steps in separating the components of mechanical mixtures and solutions on the basis of their properties (e.g., filtration of mechanical mixtures, distillation of solutions such as crude oil) •differentiate between physical and chemical properties of matter •apply the particle model of matter to explain the physical properties of the phases of matter 2. Describe solutions and solubility, solutes and solvents; and then describe how these concepts are applied to the production of prepared foods and other useful materials •provide examples of insoluble and soluble mixtures (e.g., oil and water, vinegar and water); and, in general terms, account for the difference •define, operationally, solute, solvent, solution and solubility; and express concentration in terms of mass per volume •provide examples of the effect of temperature change on solubility, and explain this effect on the basis of the particle model of matter (e.g., concentration of brines for pickling and syrups for canning) •link concentration changes and the concept of dilution to changes in the ratio of the amount of solute to the amount of solvent (e.g., investigate how concentrated products, such as orange juice, evaporated milk or instant coffee are made) •compare the volume of waste packaging produced from consumer use of the concentrated and diluted forms of products (e.g., orange juice, fabric softener), and relate this to the need for recycling and environmental preservation •identify acid and base solutions in the home, job site and laboratory (e.g., vinegar, soda pop, shampoo, battery acid, household ammonia, antacids, dish soap, hydrochloric acid, sodium hydroxide) on the basis of their general properties; i.e., they conduct electricity, change colour of acid/base indicators and neutralize one another •describe, in general terms, the pH scale as an indicator of acidity or basicity; i.e., a pH of less than 7 indicates an acid, a pH of 7 indicates a neutral solution, and a pH of greater than 7 indicates a base 3 Science 14 Unit A: Investigating Properties of Matter •describe and investigate the corrosive effects of the following environmental factors: acids, bases, salts, humidity and temperature (e.g., corrosion of iron by acid rain and spray from ocean water) •list the potential dangers of mixing common household and industrial chemicals (e.g., mixing ammonia cleaners with bleach, adding muriatic [hydrochloric] acid to caustic soda, addingwater to acid) 3. Describe the properties of elements and compounds, and use the periodic table to identify trends in properties •differentiate among metals, nonmetals and metalloids on the basis of properties (e.g., luster, conductivity, malleability, brittleness, state of matter) •use the periodic table to locate names and properties of elements •name and write chemical formulas for common elements (e.g., aluminum, copper, iron, nitrogen, hydrogen, oxygen) and simple compounds (e.g., water, glucose, table salt, carbon dioxide, iron oxide, vinegar, methane, propane), and describe the uses of elements and compounds in society •demonstrate the difference between elements and compounds on the basis of a decomposition reaction (e.g., electrolysis of water) Chapter 1 Section Objectives/Big Ideas 1.1 -Interpret safety information at work and at home. -Explain why it is dangerous to mix common household substances 1.2 -Describe what all matter has in common. -Identify the three states of matter. 1.3 2 2.1 2.2 -Determine the difference between chemical and physical properties. -Determine the difference between pure substances and mixtures. Outcomes Classify various forms of matter, including commonly used household substances, on the basis of their properties, and relate these properties to their safe use, storage and disposal Materials/Activities Chapter 1 Test -Identify how elements are organized in the periodic Describe the properties table. of elements and -Identify metals and non-metals. compounds, and use the periodic table to identify trends in properties -Identify the relationship between elements and -Chapter 2.1 & 2.2 compounds. Assignment 2.3 -Write element symbols and chemical formulas. 2.4 -Describe how elements and compounds are used in society. Chapter 2 Test 4 Science 14 3 Unit A: Investigating Properties of Matter 3.1 -Determine the difference between solutions and mechanical mixtures. 3.2 -Show how to communicate the concentration of a solution. -Examine the relationship between temperature and solubility. -Determine the difference between a solute and a solvent. -Explain why some substances are soluble and others are not. -Explore and analyze various methods of separation of solutions and mechanical mixtures. 3.3 Describe solutions and solubility, solutes and solvents; and then describe how these concepts are applied to the production of prepared foods and other useful materials -Solubility Graphing Assignment -Chapter 3 Test 4 4.1 -Determine the difference between concentrated and dilute solutions. -Explain how using concentrated solutions can help the environment. 4.2 -Identify acids and bases and their properties. 4.3 -Identify what factors influence the process of corrosion. Describe solutions and solubility, solutes and solvents; and then describe how these concepts are applied to the production of prepared foods and other useful materials -Chapter 4 Quiz UNIT A TEST 5