ß - Swansea University
advertisement

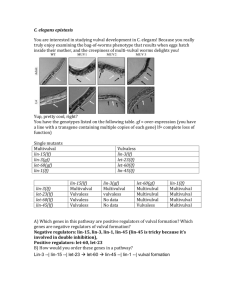
Lambda RED Recombination JIC in Norwich 11.07.2005 University of Tübingen Bertolt Gust Genomic organisation of the lambdoid bacteriophage Genes exo, bet and gam are clustered in the PL operon. red- (recombination defective) mutants were partially defective in homologous recombination in a wt-host, and grossly defective in a recA- host. The E. coli recombination system primarily restores collapsed replication forks, repairs DSB and maintains the genetic integrity of the E. coli chromosome. During the replication of , the infected cell is a hotbed of genetic exchange (hyper-rec state). 11.07.2005 Bertolt Gust How does Red stimulate homologous recombination ? linear DNA 5´ 3´ 3´ 5´ 5´ 3´ 3´ 5´ circular DNA 11.07.2005 Bertolt Gust Gam () inhibits the exonuclease V activity of the recBCD system Rec BCD linear DNA 5´ 3´ 3´ 5´ 5´ 3´ 3´ 5´ Rec BCD circular DNA Gam () binds as a dimer to the E. coli RecBCD complex and inhibits its nuclease activity 11.07.2005 Bertolt Gust Exo () binds to dsDNA ends ... linear DNA 3´ 5´ 5´ 3´ Subramanian et al., 2003 5´ 3´ 3´ 5´ circular DNA Exo () degrades linear dsDNA in 5´ to 3´direction (1kb/sec in-vitro) leaving long 3´ssDNA overhangs The active form of the protein (24kDa) is a trimer with a central hole 11.07.2005 Bertolt Gust ... and progressively generates 3´ overhangs linear DNA 3´ 3´ Subramanian et al., 2003 5´ 3´ 3´ 5´ circular DNA The entrance of the hole accommodates dsDNA, the exit diameter is the size of ssDNA 11.07.2005 Bertolt Gust Beta (ß) binds to ssDNA and mediates invasion of the ssDNA into an unbroken homologous duplex linear DNA 3´ ß ß ß ß ß ß ß ß 3´ 5´ 3´ 3´ 5´ circular DNA Beta (ß) binds to ssDNA greater than 35 nucleotides in length Beta belongs to a family of recombination proteins which include Erf protein of Salmonella phage P22, the RecT protein of the cryptic E. coli phage Rac, and the Rad52 protein of eukaryotes Beta promotes denaturation of complementary strands, strand annealing and exchange reactions 11.07.2005 Bertolt Gust RecFOR is essential for the formation of the recombination complex linear DNA RecFOR 5´ 3´ 3´ ß ß ß ß ß ß ß ß 3´ 3´ 5´ circular DNA RecFOR RecFOR replaces single-strand binding proteins (SSB) bound on ssDNA with RecA RecA stabilises complex of Bet, DNA and RecFOR 11.07.2005 Bertolt Gust RuvAB helicase-driven branch migration results in Holliday junction formation ... linear DNA Rafferty et al., 1996 RuvAB 5´ 3´ 3´ ß ß ß ß ß ß ß ß 3´ 3´ 5´ circular DNA RuvAB RuvAB recognizes a four-way junction ( Holliday junction) and catalyzes branch migration 11.07.2005 Bertolt Gust ... which can be resolved by RuvC ... linear DNA RuvC RuvC Rafferty et al., 1996 5´ 3´ 3´ ß ß ß ß ß ß ß ß 3´ 3´ 5´ circular DNA RuvC is a Holliday junction endonuclease (structure specific resolvase) 11.07.2005 Bertolt Gust ... into a recombinant molecule 11.07.2005 5´ 3´ 3´ 5´ Bertolt Gust PCR-targeting (step 1) oriT marker FRT X FRT 39bp P1 P2 target 39bp X PCR product S. coelicolor cosmid neo oriT marker FRT FRT P1 SuperCos1 bla P2 S. coelicolor cosmid neo 11.07.2005 bla Bertolt Gust PCR-targeting (step 2) target oriT marker FRT P1 FRT X P2 X S. coelicolor chromosome S. coelicolor cosmid neo SuperCos1 oriT marker FRT 11.07.2005 FRT P1 bla P2 S. coelicolor chromosome Bertolt Gust Why two-step strategy? 1. Red is efficient in E. coli 2. Recombinant cosmid-DNA can easily be confirmed by PCR, restriction analysis and/or sequencing 3. Mutagenised cosmids can be mobilised by conjugation, no need for transformation procedures 4. High frequency of double cross-overs due to long flanking sequences in a cosmid clone Disadvantages 1. Dependent upon the availability of a E. coli clone 2. Not high throughput (in comparison to transposon mutagenesis) 11.07.2005 Bertolt Gust Template cassettes for gene replacements pIJ782 P1 FRT pIJ797 P1 FRT SwaI P1 oriT aac(3)IV P2 pIJ776 P1 FRT pIJ777 P1 FRT pIJ778 P1 FRT pIJ779 P1 FRT neo neo P2 FRT FRT aadA oriT oriT P2 tet hyg P2 P2 NEW P2 oriT aadA FRT 11.07.2005 oriT FRT pIJ775 vph vph FRT P1 P2 FRT pIJ781 vph FRT P2 oriT FRT P1 FRT SwaI pIJ780 FRT oriT aac(3)IV P2 loxP P1 FRT pIJ774 oriT aac(3)IV loxP P1 FRT pIJ773 P2 P2 Bertolt Gust Template cassettes for other applications pMS80 P1 oriT aac(3)IV P2 tcp oriT aac(3)IV FRT P1 P2 fd-ter nitAp FRT egfp bla P2 tipAp FRT pIJ786 P1 aac(3)IV oriT FRT NEW aac(3)IV oriT FRT P1 int attP FRT pIJ785 tet FRT bla oriT FRT pIJ787 P2 pIJ784 bla oriT aac(3)IV bla pIJ798 bla oriT pIJ789 neoaac(3)IV neo pIJ794 neo oriT aac(3)IV neo pIJ795 neo oriT pIJ796 neo oriT aadA bla hyg NEW neo vph neo NEW S. coelicolor cosmid neo bla Herai et al., 2004. Hyper-inducible expression system for streptomycetes. PNAS 101, 14031-14035 11.07.2005 Bertolt Gust REDIRECT (Rapid Efficient Directed Recombination Time saving) Download protocol and Primer design program at http://streptomyces.org.uk/redirect/index.html To obtain the REDIRECT KIT: Mail Nicholas Bird nicholas.bird@bbsrc.ac.uk USA United Kingdom Germany Canada Spain China Korea Netherlands Japan Taiwan Argentina Belgium Finland France Israel Sweden Switzerland Taiwan 26 21 12 7 7 5 4 2 2 2 1 1 1 1 1 1 1 1 Gust, B., Chandra, G., Jakimowicz, D., Tian, Y., Bruton, C.J. and Chater, K.F. (2004) λ Red-mediated genetic manipulation of antibiotic-producing Streptomyces, Advances in Applied Microbiology 54: 107-28. Gust, B. Challis, G.L., Fowler, K., Kieser, T. and Chater, K.F. (2003) PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odour geosmin, Proc. Natl. Acad. Sci. USA 100: 1541-6 Gust, B., Kieser, T. and Chater, K.F. (2002) REDIRECT technology: PCR targeting system in Streptomyces coelicolor, John Innes Centre, Norwich Research Park, Colney, Norwich, NR4 7UH, United Kingdom 11.07.2005 Bertolt Gust Epitope tagging using REDIRECT a b aac(3)IV oriT FRT FRT P1 1. PCR ab P2 c aac(3)IV oriT FRT P1 FRT Tag P2 2. PCR ac cosmid neo 11.07.2005 bla Bertolt Gust Epitope tagging using REDIRECT a b aac(3)IV oriT FRT FRT P1 1. PCR ab P2 c aac(3)IV oriT FRT P1 FRT Tag P2 2. PCR ac cosmid neo 11.07.2005 bla Bertolt Gust Introducing point mutations using REDIRECT pIJ775 P1 aac(3)IV oriT P2 SwaI 40 bp 40 bp * X I-SceI SwaI * PCR-product P1 P2 X cosmid neo bla select for KanR CarbR transformants 11.07.2005 Bertolt Gust Single strand oligonucleotide repair (ssOR) Phage annealing proteins promote oligonucleotide-directed mutagenesis in Escherichia coli and mouse ES cells Youming Zhang, Josep PP Muyrers, Jeanette Rientjies and A. Francis Steward BMC Molecular Biology 2003, 4:1-14 • Only λ Bet is required • Strand bias: more ssOR with oligos priming the lagging strand • Efficiency of ssOR is maximum with oligos ~ 120 nt leading 5´ 3´ DnaB 5´ 11.07.2005 DNA Pol III 5´ 3´ 5´ lagging Bertolt Gust Oligo-Targeting for deleting transposon insertions 120bp dsDNA Afl II Tn5062 S. coelicolor cosmid neo bla S. coelicolor cosmid neo 11.07.2005 bla Bertolt Gust Oligo-Targeting for generation of “scar less” in-frame deletions 120bp dsDNA I-SceI Cyc2 P1 oriT aac(3)IV P2 S. coelicolor cosmid neo bla S. coelicolor cosmid neo 11.07.2005 bla Bertolt Gust Summary PCR-targeting gene knock-outs gene replacements gene fusions module swapping epitope tagging inserting point mutations promoter replacements ET-cloning integration of cosmids (attP) promoter replacements 11.07.2005 Oligo-targeting generating point mutations inserting restriction sites “scarless” mutations N-terminal epitope tagging deletion of transposon insertion Bertolt Gust Acknowledgements Department of Molecular Microbiology Celia Bruton Prof. Keith Chater 11.07.2005 Prof. Mervyn Bibb Sir David Hopwood Mark Buttner Tobias Kieser Greg Challis Helen Kieser Kay Fowler Prof. Barry Bertolt Wanner Gust