LAB-Electrolytes-characteristics of compounds
advertisement
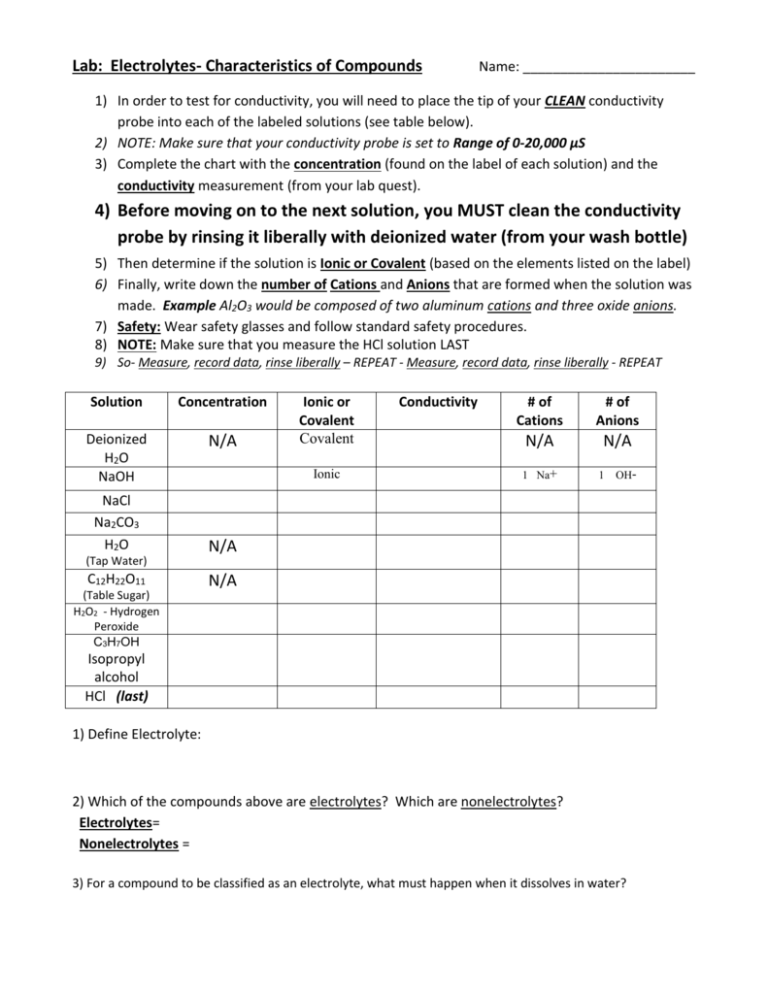
Lab: Electrolytes- Characteristics of Compounds Name: _______________________ 1) In order to test for conductivity, you will need to place the tip of your CLEAN conductivity probe into each of the labeled solutions (see table below). 2) NOTE: Make sure that your conductivity probe is set to Range of 0-20,000 µS 3) Complete the chart with the concentration (found on the label of each solution) and the conductivity measurement (from your lab quest). 4) Before moving on to the next solution, you MUST clean the conductivity probe by rinsing it liberally with deionized water (from your wash bottle) 5) Then determine if the solution is Ionic or Covalent (based on the elements listed on the label) 6) Finally, write down the number of Cations and Anions that are formed when the solution was made. Example Al2O3 would be composed of two aluminum cations and three oxide anions. 7) Safety: Wear safety glasses and follow standard safety procedures. 8) NOTE: Make sure that you measure the HCl solution LAST 9) So- Measure, record data, rinse liberally – REPEAT - Measure, record data, rinse liberally - REPEAT Solution Concentration Deionized H2O NaOH N/A NaCl Na2CO3 H2O (Tap Water) C12H22O11 (Table Sugar) H2O2 - Hydrogen Peroxide C3H7OH Ionic or Covalent Covalent Ionic Conductivity # of Cations # of Anions N/A N/A 1 Na+ 1 OH- N/A N/A Isopropyl alcohol HCl (last) 1) Define Electrolyte: 2) Which of the compounds above are electrolytes? Which are nonelectrolytes? Electrolytes= Nonelectrolytes = 3) For a compound to be classified as an electrolyte, what must happen when it dissolves in water? You will be conducting the following experiment TWICE. Once using NaCl and once with C12H22O11 a) Add approximately 200 mL of tap water to a 400 mL beaker, add the stir bar and set to low (2 or 3) b) Measure the conductivity of the tap water (without NaCl or C12H22O11) (Stirring not heat) c) Add approximately 0.5 grams of solid (NaCl or C12H22O11) and measure the conductivity of the solution. d) Repeat step “c” for five more additions of approximately 0.5 grams of solid (NaCl or C12H22O11) Table Salt (NaCl) - Ionic Added (g) Nothing added 0.5 g 0.5 g 0.5 g 0.5 g 0.5 g Total (g) 0.0 g 0.5 g 1.0 g 1.5 g 2.0 g 2.5 g Conductivity x Table Sugar (C12H22O11) - Covalent x Added (g) x Nothing added Total (g) 0.0 g Conductivity x x x x x 4) What happened to the conductivity of the solution when you continued to add NaCl? 5) Explain your answer for #4 above. 6) What happened to the conductivity of the solutions when you continued to add C12H22O11? 7) Explain your answer for #6 above. (Make your best effort, this one is kind of tricky) HINT: use the data table on the front side – look at the concentration and # of cations and # of anions 8) Why was there a difference in conductivity between NaOH and NaCl? 9) Why was there a difference in conductivity between NaCl and Na2CO3 ?






