Lesson Plan 3
advertisement

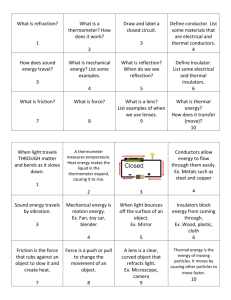
Lesson Plan 3 Conductivity Lesson Plan Grade 6 Electricity 6.2 Investigate the characteristics and applications of static electric charges, conductors, insulators, switches, and electromagnetism. [SI] e. Make predictions, based on observed patterns of events, related to the physical properties of conductors, insulators, simple circuits, and electromagnets and conduct investigations to test those predictions. f. Identify appropriate tools, instruments, and materials (e.g., bulbs, batteries, and wires) to use when investigating the properties of conductors, insulators, simple circuits, and electromagnets and use those tools and apparatus in a manner that ensures personal safety and the safety of others. g. Test the conductivity of a variety of solids and liquids, following a given set of procedures, to identify which materials are conductors and which are insulators, and draw conclusions about the types of materials that work best as conductors and which work best as insulators. Objectives: - Test different solutions and determine its conductivity by rating the brightness of the light bulb. Time: 60 minutes Background Information: Know basic information about conductors and insulators. Materials: - LED lights - Alligator clips - 9 Volt Battery - Paper clips - Beakers - Distilled/Deionized water - Tap water - Sugar - Vinegar - Coca cola - Table salt - Measuring spoons - Stirring Sticks Day Plan Set: Instructions, divide students into groups, questions – 5 minutes Exploration – See attached procedure - 35 minute Clean-up – 5 minutes Teacher Demo and questions– 15 minutes - See attached demonstration. Possible Questions: o How many grains of salt must be added to 100 mL of deioinized water to produce a noticeable glow in the LED? What percentage of salt is this? o Which solutions were the most conductive? Why do you think that is? o Are the solutions that did not light the LED light insulators? o What happens when more sugar or salt is added? Does it become more or less conductive? Explanation: You should notice that water by itself is not very conductive; that some things (baking soda and salt) make the solution a lot more conductive; while other things (like sugar) do not. What is happening is this: Really pure water is actually an insulator, and does not conduct electricity very well, so it has a high resistance. But, a lot of things that dissolve in water “dissociate”, that is, they break up into electrically charged parts (ions) that can move around. When the ions move, they conduct electricity. Substances that dissolve in water and form ions like this are referred to as “electrolytes”, because they make it possible for water to conduct electricity. Not all things that dissolve in water are electrolytes, though – the sugar will not make the water very conductive, because sugar dissolves without breaking up into ions. If your sugar did increase the conductivity a bit, it was probably because it had small amounts of some impurities that were electrolytes. The tap water should have been more conductive than the distilled water, but not as conductive as the water with salt or baking soda dissolved in it. This is because the water out of your tap is not pure, it has minerals like calcium carbonate dissolved in it. Depending on where you live, you could have “hard” water (which has a lot of dissolved minerals in it and is quite conductive), or “soft” water (which has very little dissolved minerals, and can be almost as non-conductive as distilled water). You can check the conductivities of other liquids, too, like cooking oil, vinegar, or soda pop. Also, see how adding just a little bit of baking soda or salt to water changes the conductivity, compared to adding a large amount. Resources: - http://www.andybrain.com/sciencelab/2007/12/20/multimeter-experiments-withelectricity-and-water/ http://wwwchem.csustan.edu/chem2000/EXP6/exp6a.htm