The cytoskeleton - www.bio.utexas.edu
advertisement

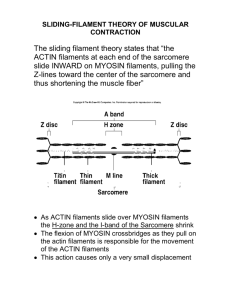
Chapter 16 The Cytoskeleton Eucaryotic cells contain protein fibers that are involved in - establishing cell shape - providing mechanical strength - cell movement - chromosome separation - intracellular transport of organelles The self-assembly and dynamic structure of cytoskeletal filaments Protein fibers form the cytoskeleton and there are 3 types of these protein filaments: - Actin filaments (also called microfilaments) - Intermediate filaments - Microtubules In addition, a large number of accessory proteins, including the motor proteins, are required for the properties associated with each of these filaments Each type of filament has distinct mechanical properties and dynamics, but certain fundamental principles are common to all. Some functions of actin filaments are: - to provide mechanical strength to the cell by forming a band under the plasma membrane - link transmembrane proteins to cytoplasmic proteins - form contractile ring during cytokinesis in animal cells - cytoplasmic streaming - generate locomotion in cells such as white blood cells and amoeba - Interact with myosin to provide force of muscular contraction Microtubules participate in a wide variety of cell activities. Most involve motion that is provided by protein “motors” that use ATP. They determine the positions of membrane-enclosed organelles and direct intracellular transport. The migration of chromosomes during mitosis and meiosis takes place on microtubules that make up the spindle fibers. Intermediate filaments provide mechanical strength and resistance to shear stress. There are several types of intermediate filaments, each constructed from one or more proteins characteristic of it. Keratins are found in epithelial cells, hair and nails Nuclear lamins form a meshwork that stabilizes the inner nuclear membrane Neurofilaments strengthen the long axons of neurons Vimentins provide mechanical strength to muscle and other cells Nature 422, 741 - 745 (17 April 2003) Intermediate filaments Defective keratins lead to the epidermolysis bullosa simplex disorder The tubulin and actin subunits assemble head-to-tail to create polar filaments The structure of a microtubule and its subunit The structure of an actin monomer and actin filament Microtubules and actin filaments have two distinct ends that grow at different rates Nucleation is the rate-limiting step in the formation of a cytoskeletal polymer The time course of actin polymerization in a test tube Filament treadmilling and dynamic instability are consequences of nucleotide hydrolysis by tubulin and actin Treadmilling occurs at intermediate concentrations of free subunits Dynamic instability Actin filaments Actin filaments nucleate most frequently at the plasma membrane and nucleation is regulated by external signals Nucleation is catalyzed by a complex of proteins that include actin-related proteins (ARPs) Differences on the sides and minus end prevent the ARPs from forming filaments on their own or with actin. Figure 16-34a Molecular Biology of the Cell (© Garland Science 2008) Figure 16-34b Molecular Biology of the Cell (© Garland Science 2008) The ARP complex nucleates actin filament growth from the (-) end, allowing rapid elongation at the (+) end The ARP complex can also attach to the side of another actin filament while remaining bound to the (-) end of the filament that it has nucleated The ARP complex nucleates filaments more efficiently when it is bound to the side of a preexisting actin filament resulting in a filament branch that grows at a 70° angle relative to the original filament Figure 16-34c Molecular Biology of the Cell (© Garland Science 2008) Actin elongation is mediated by formins Formation of actin bundles (as opposed to the gel-like branched actin networks) is induced by formins, which are able to nucleate the growth of straight, unbranched filaments that can be cross-linked by other proteins to form parallel bundles. Formins are dimeric proteins and each subunit has a binding site for an actin monomer. Figure 16-36 Molecular Biology of the Cell (© Garland Science 2008) Filament elongation is modified by proteins that bind to the free subunits Why does the soluble actin in cells not polymerized into filaments if the concentration of soluble actin is high (50-200 mM)? Although the Cc of actin monomers is 0.1 mM, the actin is not polymerized as it is bound to special proteins, such as thymosin. Actin monomers bound to thymosin are locked where they cannot associate with either the (+) end or (-) end of the actin filament. How do cells recruit actin monomers from this sequestered pool and use them for polymerization? Recruitment depends on another monomer-binding protein profilin. Profilin binds to the face of actin opposite the ATP-binding cleft. Actin-profilin can bind to the plus end of the actin filament but is unable to bind to the minus end. Figure 16-37 Molecular Biology of the Cell (© Garland Science 2008) Proteins that bind to the sides of actin filaments can either stabilize or destabilize them Tropomyosin stabilizes actin filaments by binding simultaneously to seven adjacent actin subunits in one protofilament This prevents other proteins from binding to actin Cofilin destabilizes actin filaments by forcing it to twist a little more tightly Cross-linking proteins organize assemblies of actin filaments Bundling and gel-forming proteins Polymerization of tubulin nucleated by g-tubulin ring complexes Figure 16-29 Molecular Biology of the Cell (© Garland Science 2008) Figure 16-30a Molecular Biology of the Cell (© Garland Science 2008) The centrosome Cell polarity including the organization of cell organelles, direction of membrane trafficking, and orientation of microtubules is determined by microtubule-organizing centers (MTOCs). Figure 16-30b Molecular Biology of the Cell (© Garland Science 2008) Microtubule-binding proteins (MAPs) organize microtubules and affect their stability. Some MAPs prevent or promote cytosolic microtubule polymerization; other MAPs organize microtubules into bundles or cross-link them to membranes and intermediate filaments or both. tau-green MAP2-orange Figure 16-40 Molecular Biology of the Cell (© Garland Science 2008) Figure 16-41 Molecular Biology of the Cell (© Garland Science 2008) Figure 16-44 Molecular Biology of the Cell (© Garland Science 2008) Actin-based motor proteins are members of the myosin superfamily Myosin II The myosin II bipolar thick filament Direct evidence for the motor activity of the myosin head Comparison of the domain structure of the heavy chains of some myosin types Myosin VI is unique in moving towards the minus end of an actin filament Kinesin and kinesin-related proteins The structural similarity of myosin and kinesin indicates a common evolutionary origin Dyneins are a family of minus-end directed microtubule motors They are composed of two or three heavy chains (that include the motor domain) and a variable number of light chains Two major families of dyneins – cytoplasmic dyneins and axonemal dyneins Cytoplasmic dyneins found in all eucaryotic cells – important for vesicle trafficking and localization of the Golgi apparatus near the center of the cell Axonemal dyneins are highly specialized for rapid and efficient sliding movement of microtubules that drive the beating of cilia and flagella Dynein requires the presence of a large number of accessory proteins to associate with membrane-enclosed organelles Homologs of the eucaryotic cytoskeleton in bacteria