Chapter 8
advertisement

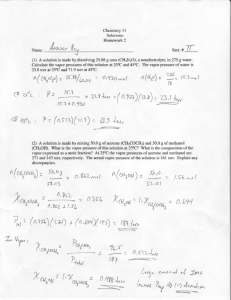
Vapor-Liquid Equilibrium (VLE) at Low Pressures Chapter 8 Why study VLE? Many chemical and environmental processes involve vapor-liquid equilibria Drying Distillation Evaporation Consider the ammonia production process discussed in Chapter 1 Recycled Product Ammonia and unreacted feed 3 moles H2 1 mole N2 N2 + 3 H2 ⇋ Controlled 2NH3 by VLE Chiller Condenses most of the ammonia Separator Bleed Stream Reactor partially NH 3 vapor+ Nconverts 2 and H2 H2 and N2 to NH3 Liquid ~15% Ammonia + N2 and H2conversion Most of what we’ve discussed so far in the course is VLE Raoult’s law is a vapor-liquid equilibrium estimating equation Henry’s law is vapor-liquid equilibrium estimating equation The biggest use of VLE analysis is in distillation Check out the great picture in the text book – page 160 Distillation is separation by boiling point Distillation Columns Simple VLE Measurement Device Othmer Still Some standard conventions used in VLE The lowest-boiling component (most volatile) is usually called species a. The next lowest is species b etc. Tables are arranged similarly Data from Table 8.1 Boiling Temp Mole Fraction Acetone in Liquid Mole Fraction Acetone in Vapor 100 0 0 74.8 0.05 0.6381 68.53 0.1 0.7301 65.26 0.15 0.7716 63.59 0.2 0.7916 61.87 0.3 0.8124 60.75 0.4 0.8269 59.95 0.5 0.8387 59.12 0.6 0.8532 58.29 0.7 0.8712 57.49 0.8 0.895 56.68 0.9 0.9335 56.3 0.95 0.9627 56.15 1 1 This data is used in examples 8.1 to 8.3, and is plotted on the next slide Acetone-Water Composition 1 Equilibrium Curve Acetone fraction in gas 0.9 0.8 0.7If all we ever dealt with were 0.6binary systems, and if tables like 0.5the one used to create this figure were available for all combinations 0.4 of species, we wouldn’t need VLE 0.3 correlations 0.2 Reference Curve 0.1 0 0 Lowest boiling point component 0.2 0.4 0.6 0.8 Acetone fraction in liquid 1 For Multispecies systems, there is no simple graph we can make The K factor can be used to help solve this problem yi Ki xi Relative volatility is an additional approach K More volatilespecies K Less volatilespecies ya xb yb x a Volatility Measures Relative volatility and "K" factors 100 Relative Volatility, 10 K, acetone 1 If is greater than 1.5 to 2 over the whole range of composition, then distillation K, is water almost always the cheapest 0.1 0 0.2 technique 0.4 0.6 0.8 separation Mole Fraction in the Liquid Phase of Acetone 1 Mathematical Treatment of LowPressure VLE The tables and graphs we’ve just looked at are easy to interpret It would be nice if we could calculate values, instead of reading them off graphs Our starting point is that the fugacity of the gas phase equals the fugacity of the liquid phase fi (1) 1 fi yi f i (1) i ( 2) (1) 0 ( 2) i i x fi For ideal gases the activity coefficient is one ( 2)0 For gases we usually choose the total pressure as the standard fugacity For liquids we usually choose the pure component partial pressure as the standard fugacity P ( 2) i Pi yi P i 0 xi Pi 0 The activity coefficient of the liquid phase ( 2) i yi P i 0 xi Pi Partial pressure of the gas In example 8.2, we use this equation to find the activity coeffients for water and for acetone at a number of concentrations Example 8.2 yacetonePTotal acetone 0 xacetonePacetone water y water PTotal 0 xwater Pwater The pure component vapor pressures are a function of temperature, and can be calculated from the Antoine equation at the appropriate boiling points. Calculated activity coefficients activity coefficient 10 acetone water 1 0 0.2 0.4 0.6 0.8 Mole fraction acetone in the liquid phase 1 Raoult’s Law yi P i 0 xi Pi yi P i xi Pi If we rearrange this equation, we find… 0 Which is the same as Raoult’s law, except for the activity coefficient. Raoult’s law applies to ideal solutions, where the activity coefficient is one. The acetone water system is not ideal, since the calculated activity coefficients are not one!! How much would we be off if we assumed ideal solution behavior for acetone? At 1 atm and Xacetone=0.05 Experimental values from Table 8.1 Calculated values using =1 Equilibrium boiling temperature 74.8 96.4 Mole fraction 0.6381 0.1656 acetone in the The calculational details vapor phase are in Example 8.3 The Four most common types of Low Pressure VLE Ideal Solution Behavior 1 Positive Deviations from Ideal 1 Solution Behavior Negative Deviations from Ideal 1 Solution Behavior Two-Liquid Phase --Heteroazeotropes Ideal Solution Behavior – Type I behavior 0.1 Activity Coefficient, 1.0 Consider a benzenetoluene system The two species are very similar chemically, and behave as an ideal solution Benzene has the lower boiling, and therefore the higher vapor pressure 10 The activity coefficient of both species is one at all concentrations – Figure 8.7b BenzeneToluene=1 At any P and T 0 0.2 0.4 0.6 0.8 1.0 Mole fraction of benzene in the liquid phase, xa Pi xi Pi 0 800 400 yi P i xi Pi 0 0 1 Pressure, torr Since the activity coefficients of both species is one, they both follow Raoult’s law 1 1200 Ideal Solution Behavior 0 0.2 0.4 0.6 0.8 1.0 Mole fraction of benzene in the liquid phase, xa Ideal Solution Behavior Figure 8.7a PToluene xToluene 407 torr PTotal PBenzene PToluene 1200 800 PBenzene 400 PBenzene xBenzene1021 torr PTotal PToluene 0 Pi xi Pi 0 Pressure, torr – at 90 C yi P i xi Pi 0 1 0 0.2 0.4 0.6 0.8 1.0 Mole fraction of benzene in the liquid phase, xa 1 0.8 1.0 Not to Scale 0.4 0.6 Equilibrium curve 0.2 Reference curve 0 For an ideal solution, the mole fraction of the most volatile component, is always higher in the gas than in the liquid Mole fraction of benzene in the liquid phase Ideal Solution Behavior Figure 8.7c 0 0.2 0.4 0.6 0.8 1.0 Mole fraction of benzene in the liquid phase, xa Ideal Solution Behavior Figure 8.7 d 95 105 115 Not to Scale 85 Bubble Point 75 Temperature, C If we heat a mixture of benzene and toluene, the temperature where it starts to boil is called the bubble point The combined partial pressures equal 1 atm 1 0 0.2 0.4 0.6 0.8 1.0 Mole fraction of benzene in the liquid or vapor phase, xa Ideal Solution Behavior Figure 8.7 d 115 Not to Scale 95 105 Dew Point 85 Bubble Point 75 Temperature, C If we cool a mixture of benzene and toluene vapor, the temperature where it starts to condense is called the dew point The combined partial pressures equal 1 atm 1 0 0.2 0.4 0.6 0.8 1.0 Mole fraction of benzene in the liquid or vapor phase, xa 115 Bubble Point Temperature at the bubble point 85 Temperature, C 95 105 Dew Point 75 Liquid Phase composition at the dew point Vapor Phase composition at the bubble point Heat a liquid mixture until it starts to boil 0 0.2 0.4 0.6 0.8 Mole fraction of benzene in the liquid or vapor phase, xa 1.0 Now let’s look at non-ideal solution behavior First let’s attack positive deviations from ideal solution behavior (Type II behavior) The activity coefficients of both (or all) species is greater than one. The acetone water system is an example of this behavior So is the isopropanol-water system shown in Figure 8.8 10 Not to Scale P=1 atm isopropanol water 1 Activity Coefficient, Notice that the behavior of each species approaches ideal (=1) as it’s concentration increases 15 Type II behavior of activity coefficients – always greater than 1 Figure 8.8 b Pure 0.8 1.0 isopropanol Mole fraction of isopropanol in the liquid phase, xa Pure water 0 0.2 0.4 0.6 i 1 Type II behavior Not to Scale Pi i xi Pi 0 1000 Pressure, torr – at 84 C Activity coefficients greater than 1 mean that the partial pressure of the vapor is greater than that predicted with Raoult’s law 800 Actual isopropanol partial pressure 600 400 Predicted with =1 200 0 0 0.2 0.4 0.6 0.8 1.0 Mole fraction of isopropanol in the liquid or vapor phase, xa i 1 Type II behavior Not to Scale Pi i xi Pi 0 1000 Pressure, torr – at 84 C Activity coefficients greater than 1 mean that the partial pressure of the vapor is greater than that predicted with Raoult’s law 800 600 Actual water partial pressure 400 200 Predicted with =1 0 0 0.2 0.4 0.6 0.8 1.0 Mole fraction of isopropanol in the liquid or vapor phase, xa i 1 Type II behavior Figure 8.8 a Scale Not to 1000 Pressure, torr – at 84 C In this case (but not every type II case) the total pressure curve (at constant temperature) displays a maximum, which produces a minimum boiling azeotrope. 800 isopropanol partial pressure 600 400 water partial pressure 200 0 0 Pi i xi Pi 0 Total pressure 0.2 0.4 0.6 0.8 1.0 Mole fraction of isopropanol in the liquid or vapor phase, xa i 1 Type II Solution Behavior Figure 8.8c 0.8 1.0 Azeotrope 0.4 0.6 Equilibrium curve 0.2 Reference curve 0 Mole fraction of isopropanol in the vapor phase When the total pressure displays a maximum, the equilibrium curve crosses the reference curve Not to Scale 0 0.2 0.4 0.6 0.8 1.0 Mole fraction of isopropanol in the liquid phase, xa i 1 Azeotropes Azeotrope Mole fraction of isopropanol in the liquid phase 0 0.2 0.4 0.6 0.8 1.0 At an azeotrope, the liquid and vapor have the same concentration Not to Scale 0 0.2 0.4 0.6 0.8 1.0 Mole fraction of isopropanol in the liquid phase, xa 100 Dew Point Curve 85 90 Azeotrope 95 Not to Scale 80 Bubble Point Curve 75 At mole fractions below the azeotrope, almost pure water, and the azeotropic composition can be distilled At mole fractions above the azeotrope, almost pure isopropanol and the azeotropic composition can be distilled Temperature , C Type II Solution Behavior i 1 0 0.2 0.4 0.6 0.8 1.0 Mole fraction of isopropanol in the liquid phase, xa Type II Solution Behavior i 1 Type II solutions do not necessarily exhibit an azeotrope For example, the acetone-water system does not Two factors contribute to this behavior Difference in boiling point Deviation from ideal behavior Type III Behavior for both species is less than 1 Similar to Type II – just insert maximum for minimum etc Consider the acetone-chloroform system Figure 8.9 b 1.0 chloroform acetone 0.4 0.6 Not to Scale P=1 atm 0.3 Activity Coefficient, Notice that the behavior of each species approaches ideal (=1) as it’s concentration increases 1.5 Type III behavior of activity coefficients – always less than 1 Pure 0 0.2 0.4 0.6 0.8 1.0 Pure chloroform Mole fraction of acetoneacetone in the liquid phase, xa i 1 Type III behavior Not to Scale 1000 Pressure, torr – at 60 C Activity coefficients less than 1 mean that the partial pressure of the vapor is less than that predicted with Raoult’s law 800 600 Predicted with =1 400 Actual acetone partial pressure 200 0 Pi i xi Pi 0 0 0.2 0.4 0.6 0.8 1.0 Mole fraction of acetone in the liquid or vapor phase, xa i 1 Type III behavior Not to Scale 1000 Pressure, torr – at60 C Activity coefficients less than 1 mean that the partial pressure of the vapor is less than that predicted with Raoult’s law 800 Predicted with =1 600 400 200 Actual chloroform partial pressure 0 Pi i xi Pi 0 0 0.2 0.4 0.6 0.8 1.0 Mole fraction of acetone in the liquid or vapor phase, xa i 1 Type III behavior Figure 8.9 a Scale Not to 1000 Pressure, torr – at 60 C In this case (but not every type III case) the total pressure curve (at constant temperature) displays a minimum, which produces a maximum boiling azeotrope. 800 Total pressure 600 Actual acetone partial pressure 400 200 Actual chloroform partial pressure 0 0 Pi i xi Pi 0 0.2 0.4 0.6 0.8 1.0 Mole fraction of acetone in the liquid or vapor phase, xa i 1 Not to Scale 0.8 Equilibrium curve Azeotrope 0.2 0.4 0.6 Reference curve 0 Mole fraction of acetone iin the vapor phase When the total pressure displays a minimum, the equilibrium curve crosses the reference curve 1.0 Type III Solution Behavior Figure 8.9c 0 0.2 0.4 0.6 0.8 1.0 Mole fraction of acetone in the liquid phase, xa i 1 Azeotropes 1.0 0.8 Equilibrium curve 0.2 0.4 0.6 Azeotrope 0 Mole fraction of acetone iin the vapor phase At an azeotrope, the liquid and vapor have the same concentration Not to Scale 0 0.2 0.4 0.6 0.8 1.0 Mole fraction of acetone in the liquid phase, xa 75 Dew Point Curve 60 65 Azeotrope 70 Not to Scale 55 Bubble Point Curve 50 At mole fractions below the azeotrope, almost pure chloroform, and the azeotropic composition can be distilled At mole fractions above the azeotrope, almost pure chloroform and the azeotropic composition can be distilled Temperature , C Type II Solution Behavior i 1 0 0.2 0.4 0.6 0.8 1.0 Mole fraction of acetone in the liquid phase, xa What controls whether the activity coefficients are greater or less than one? When >1 it indicates that the solution species are repelled by each other, or at least are more attracted to themselves than to the other species When <1 it indicates that the solution species are more attracted to each other than to their own kind More about azeotropes For a given degree of mutual attraction or repulsion, an azeotrope is more likely for species with a small difference in boiling point For pairs of compounds with the same difference in boiling point, an azeotrope is more likely for the pair whose activity coefficients deviate from one by the largest amounts Binary azeotropes between compounds with wide differences in boiling point are rare Most azeotropes are of the minimum boiling type (over 90%) Type IV Behavior Two Liquid Phase --Heteroazeotropes Type II behavior occurs when two species repel each other For a moderately strong repulsion an azeotrope forms If the repulsion is strong enough, the two liquids actually separate into two separate phases For example Consider the water and butanol system Between 65% water and 98% water, two phases occur At less than 65% water only a single phase exists At greater than 98% water only a single phase occurs Water-butanol system From 0 to 65% water one phase exists From 98% to 100% water one phase exists Between these values, two phases exist – one of 65% water and one of 98% water 2 phases 1 phase 0 0.2 0.4 0.6 Mole fraction water 0.8 1 phase 1.0 In the single phase region… water 20. 10. 1 5.0 1 1 2.0 butanol 1.0 1 Activity Coefficient, 30 The solution exhibits Type II behavior 0 0.2 0.4 0.6 0.8 1.0 Mole fraction of water in the liquid phase, xa 2 2 butanol water 1 1 800 Total Pressure 400 Partial Pressure of water Partial Pressure of nbutanol 0 Pressure, torr – at 90 C In the two phase region the total pressure is the sum of the pressure from each phase However, both phases must be exposed to the gas phase 1200 Type IV Behavior Pressure behavior 0 0.2 0.4 0.6 0.8 1.0 Mole fraction of water in the liquid phase, xa 1.0 0.8 0.4 0.6 Equilibrium Curve 0.2 Reference Line 0 The composition of the gas phase stays constant when two liquid phases are present Mole fraction of water in the vapor phase Type IV Vapor-Liquid Equilibrium 0 0.2 0.4 0.6 0.8 1.0 Mole fraction of water in the liquid phase(s), xa 120 Vapor Phase 110 Dew Point Curve 100 Vapor + L1 Vapor + L2 L2 90 L1 Bubble Point Curve L1 + L2 80 Temperature , C Call the n-butanol rich phase L1 Call the water rich phase L2 130 Type IV Bubble Points and Dew Points 0 0.2 0.4 0.6 0.8 Mole fraction of water in the liquid phase, xa 1.0 Boiling is necessary for this behavior The predicted behavior will only occur if both phases are in contact with the gas phase Gas Phase Realistically this only occurs during boiling Liquid Phase II This type of behavior is common in petroleum refining Liquid Phase I Steam Distillation If we take type IV behavior to the extreme, we consider two liquids that are essentially completely immiscible For example water and mercury In this case we are always in the two phase region P yi P Pi P 0 water P 0 Mercury The total pressure is the sum of the pure component vapor pressures Consider example 8.7 If n-butanol and water were completely insoluable, what would the boiling point be at one atm? What would the composition of the vapor be? P yi P Pi P 0 water P 0 n butanol Estimate the partial pressures of each component with the Antoine Equation log Pwater B 1657.462 A 7.94917 C T 227.02 T 1558.190 log Pn -butanol 7.838 196.8812 T Pn -butanol Pwater 760 mmHg Solve for T iteratively T=89 0C At the boiling point of 89 C PH20 = 0.67 atm Pn-butanol= 0.33 atm Compare these results to Figure 8.12 Remember that butanol and water actually do exhibit considerable solubility Distillation of the Four Types of Behavior For systems that do not have an azeotrope, distillation columns can produce practically pure products The highest vapor pressure component is separated into the overhead component The lower vapor pressure component is separated into the bottoms Gas-Liquid Equilibrium – Henry’s Law In the previous discussions, both components could exist as a pure liquid at the temperatures of interest In other words we were interested in vapor-liquid equilibria How can we extend this discussion to include gas-liquid equilibria, for species that do not condense Use Henry’s Law Our discussions so far have been about systems at low pressure At low pressures, the gas phase obeys the ideal gas law At higher pressures we’ll need to consider the fugacity coefficient in our calculations At both low and moderate pressures (up to ~1/2 the critical pressure) we won’t need to adjust our liquid phase calculations Low Pressure VLE Calculations Graphs are great to get a general idea of how systems behave, but they aren’t very accurate We need to develop a standard approach to calculate vapor-liquid equilibrium properties At low pressures the fugacity coefficients are 1 – but the liquid phase activity coefficients aren’t Estimate them using the Van Laar equation log( a ) Axb2 A xa xb B 2 log( b ) Bx a2 B xa xb A There are other estimating equations – this one is just easy to use -- the basis for the Van Laar equation is developed in chapter 9 2 The 6 Most Common VLE Calculations Find the dew point, for a known temperature Find the dew point, for a known pressure Find the bubble point for a known temperature Find the bubble point for a known pressure Isothermal flash calculations Adiabatic flash calculations These are all examples problems that can be formulated as equilibrium flash calculations Flash Calculations V Vapour F T, P Liquid L F can be a liquid, a gas or a two phase mixture This is called a flash calculation, because if the pressure is reduced enough, the liquid changes to vapor in a “flash” To solve any of these problems we need to identify our equations and unknowns -- Table 8.6 Material Balances F V L xi F yiV xi L Remember from Process Engineering that you can write n independent material balances, if you have n components Summation of mole fractions x i 1 y i 1 More equations to be solved Equilibrium yi i Pi Ki xi P Simplified version assuming ideal gas Vapor Pressure equations Antoine equation is probably the most accurate of the simple equations More equations to be solved Calculate the activity coefficients Van Laar equation is simplest analytical approach Energy balance Adiabatic flashes H F Hv H L We also need the inlet conditions Feed specification xi Temperature Pressure Let’s do some example calculations Dew Point – Example 8.9 Estimate the boiling pressure and the composition of the vapor in equilibrium with a liquid that is: 0.1238 mol fraction ethanol remainder water 85.3 oC First – Use Antoine’s equation to find the pure component partial pressures at this temperature B log Pi A C T At 85.3 C, Pwater=0.5772 atm PEtOH=1.3088 1657.462 log Pwater 7.94917 227.02 T 1554.3 log PEtOH 8.04494 222.65 T Second Find Activity Coefficients Use the Van Laar Equation log( water ) log( EtOH ) Ax 2 EtOH A xwater xEtOH B 2 Axwater 2 A xEtOH xwater B 2 Find the values of A and B for water and ethanol in Table A.7 water=1.0388 EtOH=2.9235 Next Calculate the partial pressures Pwater x 0 water water water P Pwater 0.8762 * 2.9235 * 0.5772 Pwater 0.5254 atm PEtOH 0.4737 atm PTotal 0.9991 atm Similarly for Ethanol… and… Finally Find the vapor phase mole fractions Pi yi PTotal Pwater 0.5254 y water 0.5259 PTotal 0.9991 PEtOH 0.4737 y EtOH 0.4741 PEtOH 0.9991 Pressure Specified Calculations are Similar They are harder, because the Temperature appears in the Antoine Equation They need to be solved interatively Calculational Steps Guess a Temperature Calculate the Pure Component Vapor Pressures Calculate the activity coefficients Calculate the partial pressures Calculate the total pressure, and compare to the given pressure Graphical Solution Add Figure 8.17 Bubble Point Calculations Mirror image of Dew Point Calculations Temperature specified calculations are easier Pressure specified calculations require an iterative approach Isothermal Flash Calculations Both T and P are specified The division of mass between liquid and vapor is unknown Consider Example 8.13 An ethanol-water mixture xaFeed=0.126 is brought to equilibrium at 1 atm 91.8 C Estimate the vapor fraction and the mole fraction of each species in the vapor phase Let’s Solve it Graphically First Add figure 8.19 Analytical Solution Find the pure component vapor pressures Assume a value for V/F Estimate the activity coefficients Calculate the K factors Use a material balance to find the mole fractions in the vapor and liquid phase xaF F yaV xa L xaF F yaV xa L V L xaF ya xa F F V V y a x a 1 F F Divide by F Equation 8.12 Adiabatic Flash Calculations In addition to the equations from the previous example, you need an energy balance H F HV H L You must “guess” a temperature, then perform the calculations, and finally check to see if the energy balance requirements are met Solutions using K factor approximating tools Activity coefficients are functions of T,P and x (the liquid composition) Thus, K is also a function of T,P and the liquid composition By ignoring the contribution of composition, DePreister created Figure 8.20 – an estimating tool for finding K K factor chart Colligative Properties – Another application of Raoult’s Law Boiling Point elevation Freezing Point depression Osmosis (Chapter 14) Predictable using Raoult’s Law What happens as the concentration of the solvent approaches 1? The solvent activity coefficient, , approaches 1 Thus for dilute solutions of anything the solution obeys Raoult’s law The identity of the solute doesn’t matter, as long as it’s a dilute solution and it’s not volatile!! Boiling Point Elevation When the solute has a very high boiling point, the solution vapor pressure only depends on the solvent 0 PT Psolvent Psolute PT xsolvent P 0 solvent Boiling Occurs when the vapor pressure equals the pressure of the surroundings Example 8.15 One mole of sucrose (MW=342.3 g/mole) is dissolved in 1000 g of water What is the vapor pressure of this Boiling solution at 100 0C? PT 0.982 *1 atm 0.982 atm won’t occur, unless you’re at an altitude above sea level What temperature will cause boiling? First – determine what pure component vapor pressure is required PT xsolvent P 0 solvent 1atm 0.982 P P 0 solvent 0 solvent 1.018atm From the steam tables we find T=100.51 0C – or a boiling point elevation of 0.51 0C We can approximate the behavior of this solution at low concentrations of solute The change in boiling point is directly proportional to the molality of the solution Tboiling K b m Figure 8.22 Freezing Point Depression Just as adding a non-volatile solute increases the boiling point, it also decreases the freezing point See Example 8.16 T freezing K f m