Barbara Melosky, MD, FRCPC
advertisement

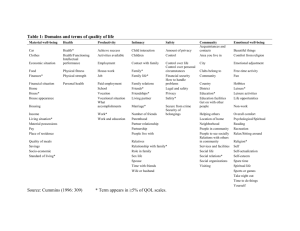
The Importance of PRO/HRQoL Measures in Patient Management and Their Inclusion in Oncology Clinical Studies Barbara Melosky, MD, FRCPC Objectives • Discuss increasing importance of PRO/QoL measures in influencing the treatment of patients with cancer • Discuss the need to include PRO/QoL endpoints in clinical trials • Highlight QoL outcomes in Lung Cancer Trials Endpoints and Treatment Relationships and Role of Patient Reported Outcomes (PROs) Malignancy Survival Quality of Life Tumour Response & Side Effects Treatment Quality of Life and PRO Evaluation Increasing Importance • Highly Symptomatic Disease – Survival and response data reveal only part of the results that are important to patients, families, and health care professionals • Treatments and Agents Vary in their Side-Effects and Risk Profiles – Balancing patient-experienced benefit and risk is needed • Meaningful Survival Differences are Uncommon Symptom Control Is Important to Patients 68% of patients said they would choose chemotherapy only if it improved symptoms Silvestri G et al. BMJ. 1998;317:771-775. Quality of Life and PROs: Questions 1. Can we DEFINE quality of life? 2. Can we MEASURE quality of life? 3. Can we agree on how to ANALYSE quality of life results? 4. Can we PRESENT quality of life findings in a clear and useful way? What Is QoL? What Is QoL? “Optimal levels of physical, role and social function, including relationships and the perception of health, fitness, life satisfaction and well-being.” – Bowling, 1995 What Is QoL? • WHO: “Health is not only the absence of infirmity and disease, but also a state of physical, mental and social well-being.” • Multiple domains: – Physical, cognitive, emotional and social functioning, pain, sexual functioning, health perceptions, and symptoms about nausea and fatigue What Is QoL? • “The goodness of life” • Multi-dimensional • Includes hope/hopefulness • As related to health (not housing, income, environment, etc) • Patient’s perspective • Fundamental Principle: QoL IS ASSESSED BY THE PATIENT Why Are We Interested in PRO/QoL in Clinical Trials? Why Are We Interested in QoL/PROs? • PROs following treatment are considered to be important supportive measures for patients • PROs should be viewed as components of the total value of a treatment and, together with other cancer endpoints, provide a comprehensive picture of the benefits and risks of anticancer therapies • This has been adopted by the FDA and EMEA Why Are We Interested in Quality of Life (QOL)? • The FDA has stated that efficacy with respect to overall survival and/or improvements in QOL might provide the basis for drug approval O’Shaughnessy et al. J Clin Oncol. 1991;9:2225-2232. PROs: Rationale and Need in Testing Anticancer Agents • Health care professionals are not accurate in evaluating subjective or palliative benefits associated with anti-cancer treatments, when compared with patient self-reports • PROs are often reported by patients as improved with less than major responses to treatment—even with only stable disease…response rates underestimate patient-reported benefit • The balance between symptom improvement and toxicity, or the effects of delayed progression summarised in many PRO measures, cannot be consistently predicted by other biomedical endpoints Why is QOL and Increasingly Frequent Outcome in Clinical Trials? • Disease-centered outcomes (response rates, cause-specific survival) are not the only clinically relevant outcomes • Toxicity has traditionally been assessed from the view-point of medical staff • Patient-centered outcomes have become an important measure of the patient experience of their illness and treatment NCIC CTG NCIC GEC How Do You Measure QoL? Measuring QoL How are you feeling today? Happy Miserable Measuring QoL • Hardest part! • Determine QoL objective • Choose instrument to measure QoL – Reliable, valid, responsive, feasible – Global measures, disease-specific measures, symptom checklists • Select assessment time points • Develop analysis plan Measurement • Look for measures that are proven to be – VALID – RELIABLE • Validity: does measure actually measure the construct it is intended to measure? • Reliability: how much closer does our measure get to the “true” score? (ranges from 0 to 1) Validity • Does the questionnaire really measure QOL? • Face and content validity – Do the questions make sense? – Are they relevant? – Is the administration and scoring sensible? – Criterion validity • Compare to a “Gold Standard” NCIC CTG NCIC GEC Reliability • Does the questionnaire produce reproducible results? – Internal consistency (similar items score similarly, eg. Chrobach’s alpha) – Test-retest reliability (5-7 days) – ideally concordance (ICC) rather than correlation – Longer questioners are more reliable NCIC CTG NCIC GEC Latent Variables • QoL is, by definition, a “latent variable”: it cannot be directly measured • We measure it using “symptoms” of QoL • Statistical methods help us make inferences about state of QoL via the symptoms – Develop models/scales for measuring QoL – We can maximise reliability and evaluate validity • Issues to consider: – What if our “symptoms” are not tapping into QoL like we think? – What if patients’ perceptions of the questions we ask are different? – How can we find out about these things???? Latent Variable Depiction Have you felt nauseated? Have you had problems sleeping? Quality of Life Have you had pain? Have you lacked appetite? Have you felt depressed? Issues to Consider • The 5W’s: Who, What, When, Where, Why, How – Who are the patients (cancer type, age, etc) – What are their concerns or issues – When & Where will QOL be measured – Why measure QOL – How? Self-completed, computerized, interview NCIC CTG NCIC GEC Who and What? • Consider cancer type, gender, age, level of education, stage, treatment, and point disease course • Review literature or interview patients about their issues and concerns • Are there existing, validated instruments? • Consider emotional, social, cognitive, role fulfillment and spiritual issues as well as physical condition NCIC CTG NCIC GEC When and Where? • Cross-sectional vs. longitudinal design • Do you want to describe a state, or measure change? • Beware of pitfalls of missing or untimely data • Respondents may be more comfortable in their own homes, but the clinic may be more practical • Timing with respect to doctor’s visits NCIC CTG NCIC GEC How? Administration • Self-completion is the gold standard • Interviews must be standardized • Use of computers is promising • Use of proxy information is difficult NCIC CTG NCIC GEC How Important is it to Complete QOL Questionnaires? • Extremely important!!! • The biggest problem with analizing QOL information from clinical trials is missing data – Are pts whose QOL data are missing different from pts supplying QOL data? Or is QOL data missing because pts are sicker than those providing info? • Analysis can try to account for missing data but it is best trying to prevent missing data NCIC CTG NCIC GEC Example of QOL “Result” • EORT QLQ-C30 +3 Instrument • Domain: Global quality of life • Patient questionnaires items: How would you rate your overall health during the past week? 1 2 3 4 5 6 7 8 Very Poor 9 10 Excellent How would you rate your overall quality of life during the past week? 1 Very Poor NCIC CTG NCIC GEC 2 3 4 5 6 7 8 9 Excellent 10 Example of QOL “Result” NCIC CTG NCIC GEC Courtesy of Barbara Melosky, MD Same Data Presented Differently NCIC CTG NCIC GEC Courtesy of Barbara Melosky, MD Percent of Patients Same Data Presented Differently NCIC CTG NCIC GEC Courtesy of Barbara Melosky, MD Choosing Your Instrument • “Tailor Made” Instruments – Quick and simple – Standardised but targeted to disease – Validated, normed to trial population – Select subsets of off-the-shelf instruments • Home-Made Instruments – Often too long – Often not validated or ‘normed’ or field-tested in the patient population of interest Assessment Instruments a. CARES-SF (Schag 1991) – 59 item scale which measures rehabilitation and quality of life in patients with cancer. This has been modified to the HIV Overview of Problems Evaluation Systems (HOPES, Schag 1992). b. City of Hope Quality of Life, Cancer Patient Version (Ferrell 1995) – a 41 item ordinal scale representing the four domains of quality of life including physical well being, psychological well being, and spiritual well being. c. Daily Diary Card-QOL (Gower 1995) – a self-administered card for use in cancer clinical trials that has been shown to demonstrate short-term changes in quality of life related to symptoms induced by chemotherapy. d. EORTC QOL-30 (Aaronson 1993) – this instrument is composed of modules to assess quality of life for specific cancers in clinical trials. The current instrument is 30 items with physical function, role function, cognitive function, emotional function, social function, symptoms, and financial impact. e. FACT-G (Cella 1993) – a 33 item scale developed to measure quality of life in patients undergoing cancer treatment. f. FLIC (Finkelstein 1988) – a 22 item instrument which measures quality of life in the following domains: physical/occupational function, psychological state, sociability, and somatic discomfort. This scale was originally proposed as an adjunct measure to cancer clinical trials. g. Southwest Oncology Group Quality of Life Questionnaire (Moinpour 1990) – a scale developed for cancer patients incorporating questions from various function, symptoms, and global quality of life measures. QALY • “QALY” = Quality Adjusted Life Years • Often interested in whether or not survival with poor quality of life is better than death without suffering • Example: – Cancer: many patients would rather not get toxic therapies and have more enjoyable end of life • The general idea is to down-weight time spent in periods of poor quality of life • Methodologically challenging: – How to determine the weights? – Different settings might need different weights Quality Adjusted Survival QTWIST: Quality-Adjusted Time Without Symptoms of Disease and Toxicity • Evaluate therapies based on both quantity and quality of life through survival analysis • Based on QALYs Quality of Life for Individual – Define QOL health states, including one with good health (minimal symptoms) – Patients progress through health states and never back-track – Partition the area under the Kaplan-Meier Curve and calculate the average time spent in each clinical health state – Compare treatment regimens using weighted sums durations, weights are utility based Example: 5 year survival 0.6 1 08 . 2 0.6 1 0.2 1 3 3 adjusted years of life Compare the average QTWIST in two treatment groups. Could be that on treatment A, people live longer, but QOL is worse. Lung Cancer Non–Small Cell Lung Cancer • Quality of life at baseline: Influence on survival • Prospective analysis of 673 patients at 30 centres Percent Surviving* 100 80 60 40 20 0 0 2 4 6 8 10 12 14 16 Months Lower QoL *P=0.0001, using the LCSS quality of life instrument. Higher QoL 18 20 22 24 How Do Lung Cancer–Related Symptoms Affect Patients’ Quality of Life? • 90% of patients with advanced NSCLC experience two or more disease-related symptoms and high levels of associated psychological distress Cough Dyspnoea (shortness of breath) General symptoms of fatigue, pain & anorexia Tanaka T, Gotay CC. Acad Med. 1998;73:1003-1005; Bottomley A. Oncologist. 2002;7:120-125. Quality of Life Instruments: Lung Cancer–Specific Various Questionnaires Measure PROs Questionnaire EORTC Core Quality of Life Questionnaire (QLQ-C30) EORTC lung cancer supplement (QLQ-LC13) EuroQol EQ-5D Functional Assessment of Cancer Therapy – Lung (FACT-L) Lung Cancer Symptom Scale (LCSS) - Patient (9 items) & Observer (6 items) Forms EORTC Core Quality of Life Questionnaire (QLQ-C30) • Comprises 30 questions • Five functional scales – Physical, role, cognitive, emotional, social • Three symptom scales – Fatigue, pain, nausea/vomiting • A global health status/QoL scale • A number of single items – Dyspnoea, loss of appetite, sleep disturbance, constipation, diarrhoea, financial impact • Four-point scale from 1 (not at all) to 4 (very much) • Takes 11–12 minutes to complete, on average Available at: http://groups.eortc.be/qol/eortc-qlq-c30. EORTC Lung Cancer Supplement (QLQ-LC13) • Disease-specific modular questionnaire that should always be complemented by the QLQ-C30 • Comprises 13 items across two domains/categories – Lung cancer-related symptoms, treatment side effects • Four-point scale from 1 (not at all) to 4 (very much) • Takes less than 10 minutes to complete, on average Available at: http://groups.eortc.be/qol/sites/default/files/img/specimen_lc13_english.pdf. EuroQol EQ-5D Questionnaire • Consists of five domains and a visual analogue scale (EQ VAS) – Mobility, self-care, usual activity, pain, anxiety/depression • Each dimension has three levels – No problems – Some problems – Extreme problems • Takes 8 minutes to complete, on average Available at: http://www.euroqol.org/eq-5d-products/how-to-obtain-eq-5d.html. FACT-L (Version 4) • Comprises 36 items across five domains/categories • Category domains – Physical, social/family, emotional, and functional well-being; lung cancer subscale (symptoms, cognitive function, regret of smoking) • Five-point scale from 0 (not at all) to 4 (very much) • Takes 11–12 minutes to complete, on average Available at: www.facit.org/LiteratureRetrieve.aspx?ID=42271. Transforming Questionnaire Answers Into Scores • For each scale/item a linear transformation is applied to standardise the raw score to a range from 0 to 100 – 100 = best possible function/QoL for functional scales – 100 = highest burden of symptoms for symptom scales and symptom items • A 10-point change in an item/domain is perceived to be clinically meaningful Examples of QoL Results in Lung Cancer Trials PRO Assessments in First-Line EGFR Mutation-Positive Clinical Trials Trial Treatments IPASS Gefitinib versus carboplatin + paclitaxel Mok T et al. N Engl J Med. 2009;361:947-957 QoL assessments FACT-L and FACT-TOI Methodology Outcomes Randomisation, Week 1, every 3 weeks until day 127, once every 6 weeks from Day 128 until disease progression, and when the study drug was discontinued Significantly more patients in the gefitinib group than in the carboplatin/paclitaxel group had a clinically relevant improvement in QoL and by scores on the FACT-TOI. Rates of reduction in symptoms were similar PRO Assessments in First-Line EGFR Mutation-Positive Clinical Trials (cont’d) Trial Treatments EURTAC Erlotinib versus cisplatin + docetaxel or gemcitabine QoL assessments Completion of the lung cancer symptom scale Rossell R et al. Lancet Oncol. 2012;13:239-246; Methodology Outcomes Baseline, every 3 weeks, end of treatment visit and every 3 months during follow-up Insufficient data collected for any analysis to be done – due to low compliance PRO Assessments in First-Line EGFR Mutation-Positive Clinical Trials (cont’d) Trial Treatments QoL assessments LUXLung 3 Afatinib versus cisplatin + pemetrexed LUXLung 6 Afatinib versus gemcitabine + cisplatin Methodology Outcomes EORTC QLQC30, EORTC QLQ-LC13 Baseline, every 3 weeks until disease progression Afatinib improved lung cancer-related symptoms and QoL, and delay of deterioration of symptoms compared with chemotherapy EORTC QLQC30, EORTC QLQ-LC13 Baseline, every 3 weeks until disease progression Afatinib improved lung cancer-related symptoms of cough, dyspnoea and pain, and global health status/QoL compared with chemotherapy Sequist LV et al. J Clin Oncol. 2013;31:3327-3334; Yang JC et al. J Clin Oncol. 2013;31:3342-3350; Geater SL et al. J Clin Oncol. 2013;31(suppl):Abstract/Poster 8061. IPASS: Rates of Improvement in Scores for Quality of Life and Symptoms Patients with Sustained Clinically Relevant Improvement (%) Gefitinib (N=590) 60 50 Odds ratio, 1.34 (95% CI, 1.06-1.69) P=0.01 Carboplatin plus paclitaxel (N=561) Odds ratio, 1.78 (95% CI, 1.40-2.26) P<0.001 Odds ratio, 1.13 (95% CI, 0.90-1.42) P=0.30 51.5 48.0 46.4 48.5 40.8 40 32.8 30 20 10 0 Total FACT-L Mok T et al. N Engl J Med. 2009;361:947-957. TOI LCS All Patients Had Low Baseline Symptom Burden Patient symptom scores at baseline in LUX-Lung 3 and LUX-Lung 6 LUX-Lung 3 Afatinib LUX-Lung 6 Pem/Cis Afatinib Gem/Cis EORTC QLQ-LC13 or QLQ-C30 Item/domain Mean Score (SD) n Mean Score (SD) n Mean Score (SD) n Mean Score (SD) n Cougha (Q1/QLQ-LC13) 35.6 (25.6) 224 32.7 (24.8) 109 37.3 (23.8) 233 29.4 (25.9) 109 Dyspnoeaa (domain: Q3-Q5/QLQ-LC13) 22.1 (19.2) 224 24.9 (24.1) 111 25.3 (19.0) 232 24.0 (20.6) 109 Short of breath (Q8/QLQ-C30) 27.1 (23.8) 224 25.5 (29.1) 111 25.9 (23.1) 229 22.9 (23.0) 109 Paina (domain: Q9, Q19/QLQ-C30) 25.5 (23.5) 224 23.6 (25.6) 111 24.4 (21.8) 232 22.8 (23.1) 109 Pain in chest (Q10/QLQ-LC13) 22.3 (23.6) 224 19.2 (24.0) 111 22.1 (22.2) 231 21.3 (22.5) 108 Pain in arm or shoulder (Q11/QLQ-LC13) 18.9 (24.3) 224 12.3 (19.6) 111 21.7 (25.7) 230 16.8 (21.6) 109 Pain in other parts (Q12/QLQ-LC13) 21.6 (26.4) 213 18.8 (26.0) 101 19.2 (22.9) 220 17.8 (21.3) 103 aPrespecified NSCLC symptoms. Data on file. Boehringer Ingelheim. Sequist et al. ESMO 2012. Abstract 1229PD. LUX-Lung 3: All Patients Had Low Baseline Symptom Burden Patient symptom scores at baseline EORTC QLQ-LC13 or QLQ-C30 item/domain Afatinib Cisplatin/ pemetrexed Mean score (SD) n Mean score (SD) n Cough* (Q1/QLQ-LC13) 35.6 (25.6) 224 32.7 (24.8) 109 Dypnoea* (domain: Q3-Q5/QLQLC13 22.1 (19.2) 224 24.9 (24.1) 111 Short of breath (Q8/QLQ-C30) 27.1 (23.8) 224 25.5 (29.1) 111 Pain* (domain Q9, Q19/QLQC30) 25.5 (23.5) 224 23.6 (25.6) 111 Pain in chest (Q10/QLQ-LC13) 22.3 (23.6) 224 19.2 (24.0) 111 Pain in arm or shoulder (Q11/QLQLC13) 18.9 (24.3) 224 12.3 (19.6) 111 Pain in other parts (Q12/QLQLC13) 21.6 (26.4) 213 18.8 (26.0) 101 • Compliance with questionnaires was >85% during the treatment period *Prespecified non-small cell lung cancer symptoms. Yang JC et al. J Clin Oncol. 2013;31:3342-3350. LUX-Lung 3: Percentage of Patients Who Improved in the Symptoms of Cough, Dyspnoea and Pain LUX-Lung 31 P 0.244 Cough (Q1 from QLQ-LC13) 0.010 Dyspnoea (Q3-5 from QLQ-LC13) 0.977 Dyspnoea rested (Q3 from QLQ-LC13) 0.266 Dyspnoea walked (Q4 from QLQ-LC13) 0.011 Dyspnoea climbed stairs (Q5 from QLQ-LC13) Short of breath (Q8 from QLQ-C30) <0.001 Pain (Q9, Q19 from QLQ-C30) 0.051 0.010 Have pain (Q9 from QLQ-C30) 0.127 Pain affecting daily activities (Q19 from QLQ-C30) 0.018 Pain in chest (Q10 from QLQ-LC13) Pain in arm/shoulder (Q11 from QLQ-LC13) 0.010 Pain in other parts (Q12 from QLQ-LC13) 0.170 0 20 40 60 Patients (%) 80 Afatinib (n=230) Cisplatin/pemetrexed (n=115) *Prespecified NSCLC symptoms. European Organization for Research and Treatment of Cancer scores improved by ≥10 points. Yang JC et al. J Clin Oncol. 2013;31:3342-3350. LUX-Lung 3: Afatinib Delayed the Worsening of Cough Estimated probability 1.0 0.8 Afatinib N=230 Cis/Pem N=115 No. of events 78 44 mTTD (mo) NE 8.0 0.60 (0.41 to 0.87) P=0.007 HR (95% CI) 0.6 0.4 0.2 Afatinib Pemetrexed/cisplatin 0 0 3 6 9 12 15 18 21 24 27 8 0 2 0 0 0 Time to deterioration (months) Number at risk: Afatinib 230 Pemetrexed/ 115 cisplatin Yang JC et al. J Clin Oncol. 2013;31:3342-3350. 166 62 134 27 104 14 68 5 42 3 26 0 LUX-Lung 3: Afatinib Delayed the Worsening of Dyspnoea Afatinib N=230 Cis/Pem N=115 No. of events 118 67 mTTD (mo) 10.3 2.9 Estimated probability 1.0 0.8 0.6 0.68 (0.50 to 0.93) P=0.015 HR (95% CI) 0.4 0.2 Afatinib Pemetrexed/cisplatin 0 0 3 6 9 12 15 18 21 24 27 3 1 1 0 0 0 Time to deterioration (months) Number at risk: Afatinib 230 Pemetrexed/ 115 cisplatin Yang JC et al. J Clin Oncol. 2013;31:3342-3350. 128 48 106 20 84 11 58 5 34 3 20 2 LUX-Lung 3: Afatinib Delayed the Worsening of Pain Estimated probability 1.0 0.8 Afatinib N=230 Cis/Pem N=115 No. of events 144 72 mTTD (mo) 4.2 3.1 HR (95% CI) 0.83 (0.62 to 1.10) P=0.19 0.6 0.4 0.2 Afatinib Pemetrexed/cisplatin 0 0 3 6 9 12 15 18 21 24 27 4 0 1 0 0 0 Time to deterioration (months) Number at risk: Afatinib Pemetrexed/ cisplatin Yang JC et al. J Clin Oncol. 2013;31:3342-3350. 230 113 115 49 79 22 55 9 40 3 24 0 17 0 LUX-Lung 3: Time to Deterioration for Individual Components of Cough, Dyspnoea and Pain-Related Items HR (95% Cl) P Value Cougha (Q1 from QLQ-LC13) 0.60 (0.41-0.87) 0.007 Dyspnoeaa (Q3-5 from QLQ-LC13) 0.68 (0.50-0.93) 0.015 Dyspnoea rested (Q3 from QLQ-LC13) 0.82 (0.55-1.21) 0.304 Dyspnoea walked (Q4 from QLQ-LC13) 0.62 (0.44-0.89) 0.008 Dyspnoea climbed stairs (Q5 from QLQ-LC13) 0.64 (0.46-0.91) 0.011 Short of breath (Q8 from QLQ-C30) 0.48 (0.33-0.68) <0.001 0.83 (0.62-1.10) 0.191 Have pain (Q9 from QLQ-C30) 0.88 (0.64-1.22) 0.436 Pain affecting daily activities (Q19 from QLQ-C30) 0.94 (0.69-1.28) 0.687 Pain in chest (Q10 from QLQ-LC13) 0.65 (0.45-0.94) 0.023 Pain in arm/shoulder (Q11 from QLQ-LC13) 0.94 (0.65-1.34) 0.721 Pain in other parts (Q12 from QLQ-LC13) 1.09 (0.78-1.52) 0.621 Paina (Q9, Q19 from QLQ-C30) 4/16 Favours afatinib 1 Favours cis/pem 4 Afatinib delayed time to deterioration for the individual items of dyspnoea and pain, compared with cisplatin/pemetrexed *Prespecified NSCLC symptoms. Yang JC et al. J Clin Oncol. 2013;31:3342-3350. 16 LUX-Lung 3: Afatinib Associated With Improved QoL Difference in mean scores over time (longitudinal analysis) Mean Treatment Difference (95% CI) Mean Treatment Difference (95% CI) P Value Global health status –3.18 (–5.75 to –0.61) 0.015 Physical (QLQ-C30: Q1-5) –4.80 (–7.47 to –2.13) <0.001 Role (QLQ-C30: Q6-7) –4.40 (–7.40 to –1.40) 0.004 –0.87 (–3.20 to 1.46) 0.462 Cognitive (QLQ-C30: Q20 and 25) –3.16 (–5.47 to –0.85) 0.007 Social (QLQ-C30: Q26-27) –1.11 (–3.94 to 1.72) 0.442 Global health status/QoL (QLQ-C30: Q29-30) Functional scales Emotional (QLQ-C30: Q21-24) –10 –8 –6 –4 –2 Favours afatinib 0 2 4 6 8 10 Favours cisplatin/pemetrexed • Afatinib significantly improved the global health status/QoL; role, cognitive and physical functioning were significantly improved compared with cisplatin/pemetrexed Yang JC et al. J Clin Oncol. 2013;31:3342-3350. LUX-Lung 6: Percentage of Patients Who Improved in the Symptoms of Cough, Dyspnoea and Pain Afatinib Patients with improvement (%) 80% 80 Gem/Cis P=0.0003 P<0.0001 70% 70 P=0.0029 P=0.0061 60% 60 P=0.0211 50% 50 P=0.1395 P=0.0924 40% 40 30% 30 20% 20 10% 10 0% 0 Cough Dyspnoea Short of breath Pain Pain in chest Pain in Pain other arm/shoulder • Significantly higher number of patients had symptom improvement with afatinib for the prespecified symptoms of cough, dyspnoea and pain Geater SL et al. J Clin Oncol. 2013;31(suppl):Abstract/Poster 8061. LUX-Lung 6: Percentage of Patients Who Improved in Global Health Status/QoL and Functional Scales Afatinib Patients with improvement (%) 70 Gem/Cis P<0.0001 60 P=0.0007 P<0.0001 P=0.0129 50 P=0.3828 P=0.1003 40 30 20 10 0 Global health status Physical functioning Role functioning Emotional functioning Cognitive functioning Social functioning • Significantly higher number of patients had an improvement in global health status/QoL and physical, role and social functioning Geater SL et al. J Clin Oncol. 2013;31(suppl):Abstract/Poster 8061.