Y2 Q2 Chemistry Labs, Rhetoric Level, by George Schmitz
advertisement

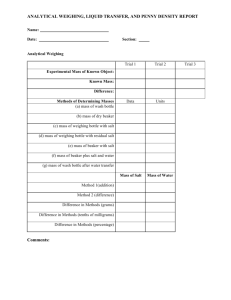
Lab IV: Making Sense of Dense Cents: Making Density Measurements Objective: 1. Distinguish physical and chemical properties of matter 2. Measure mass by displacement and a scale balance 3. Measure volume by displacement and algebraic arithmetic 4. Learn about significant figures in density calculations Introduction: The universe is comprised of approximately 92 naturally occurring elements*. Atoms of these elements combine in various ways to form compounds. How atoms may be arranged in compounds, in chemical reactions, depends on the chemical properties of the elements. Flammability, or the ability to burn in the presence of oxygen, is one example of a chemical property, but burning changes the substance. On the other hand, physical properties of a substance may be observed without changing the identity and composition of the substance. Density, the quotient of mass divided by volume, is an important physical property that is relatively easy to determine. The use of metal coins has an extensive history of approximately 2,500 years. Coinissuing governments occasionally change the metals used to make the coins to keep up with supply and demand that affect the value of the coins. For example, the use of silver and gold in U.S. currency was once critical to the value of the coins, but the value of these metals is now so high that they are used only in minting special edition collector’s coins. Less valuable metals are used to mint common coins (pennies, nickels, quarters) in circulation. Pennies are no exception. In 1982, the U.S. Mint changed the metal composition of the Lincoln cent. In this experiment, students will determine the densities of pre-1982 and post-1982 pennies (Pennies minted in 1982 are not all the same, so do not use this year.) To do this, students will use two methods each of measuring mass and measuring volume. They will also learn about significant figures, which reflect the levels of accuracy and precision in performing the experiment. * Approximately 118 elements have been found, but many were observed only in an artificial laboratory setting. Two of the 92 might also not be “naturally occurring”. Terms: Accuracy, Precision, Atoms, Elements, Compounds, Chemical Properties, Physical Properties, Density, Mass, Volume, Significant Figures Supplies: • Pennies, pre-1982 and post-1982 (up to 10 each category per student), clean and in fair condition • Vernier Caliper or Digital Caliper (small, inexpensive ones are fine, or might borrow some from a mechanic), with metrics • Metric kitchen or jewelry scale, or balance scales with accuracy at least within +/- 1g • Metric ruler • Small plastic tumblers or medicine cups that will float in water if holding a penny 1 • Graduated beakers that will float the tumbler or medicine cup • Graduated cylinder that will easily fit a penny without getting stuck • Distilled water • Charts to have handy: • Periodic table of the elements • Density Periodic Table • Periodic table showing atomic radii trends Teacher Prep: Parts A, B, C, and D can be done in any order. You may arrange them as stations. Part A: Measuring mass by Archimedes’ principle of flotation Teacher • (Do your students know the story of Archimedes of Syracuse who shouted “Eureka!”?) • Principle of Flotation: A floating body displaces a volume that has an equivalent mass. The water level increases by the how much the boat weighs. • Students must follow these rules about significant figures. Rule 1: The number of significant figures in the result is limited by the least certain measurement. Addition or subtraction example: limited by digit of smallest unit 13.0 g - 4g 9g 4 g is the least precise measurement with a value in the ones place but not a decimal place, so the result may not have a decimal place. Multiplication or division example: keep fewest number of sig. figs Area = 5 cm 0.563 cm = 3 cm2 (“5 cm” is the least certain measurement. The actual result 2.815 cm must be rounded to 3 cm2 to keep only one sig. fig. as in “5 cm”.) Rule 2: Zeros are significant if they are: i.) between non-zero digits 1007 has four sig. figs.; 1700 has one sig. fig. ii.) to the right of the decimal place and to the right of a non-zero digit 0.0070 has two sig figs; 0.007 has one sig. fig. iii.) left of the decimal and a decimal is written 100 has one sig. fig.; 100. has three sig. figs. Rule 3: Exact numbers may be assumed to have an infinite number of significant figures. –e.g. two pennies = 2.0000000000 etc. Students 2 1. Prepare a boat (small cup) to hold a pre-1982 penny(ies) in a graduated cylinder. Place the empty boat into a graduated beaker partially filled with distilled water. Do not allow the boat to touch the side of the beaker when you make your readings! Record the initial volume. ________ cubic centimeter (cm3) Note: 1 cm3= 1 mL You may estimate the final digit. For example, if your graduations are in units of 50mL, then you may estimate to the ones position e.g. 52 mL, but keep in mind that the last digit is uncertain. 2. Add the penny (or a specific number of pennies) and record the final volume. ________ cm3 (Warning: If the boat sinks or takes in any water, you have to dry the boat & pennies and start over!) 3. Subtract the initial volume from the final volume. _____ cm3 4. Divide the result above by the number of pennies you put in the boat. ____ cm3 5. Use the density of water at 20˚C (0.99821 g/cm3) to determine the mass of your penny with the equation below. # mL water displaced density of water = # grams Record the mass of your penny. ____ g 6. Repeat Steps 1-5 for post-1982 pennies. Initial volume: _____ cm3 Final volume: _____ cm3 Change in volume: _____ cm3 Volume per penny: _____ cm3 Grams per penny: _____ g Part B: Determine mass using a scale or balance. Instruct students in the use of your kitchen scale or balance. If your scale does not measure in 0.1 g units or smaller, it may be necessary to measure 10 pennies at a time and divide to obtain an average mass. If the scale is electronic, the final digit (furthest to the right) is already an estimate. If the scale is analog, they may estimate one final digit (reading between the lines). Pre-1982 penny ____g Post-1982 penny ___g Part C: Determine volume by displacement 1. Partially fill a graduated cylinder with distilled water. Record the volume from the bottom of the meniscus. ___cm3 2. Drop one or a specific number of pre-1982 pennies into the cylinder. Record the new volume. If you added more than one penny, then first divide by the total 3 number of pennies. ___cm3 3. Repeat 1-2 for post-1982 pennies. Part D: Determine volume by dimensions If using Vernier or digital calipers, then please instruct your students in its use. Calipers usually estimate the final digit for you. If calipers are not available, then allow students to use metric rulers. They will be able to estimate to 1/10th of a millimeter. 1. Measure the diameter of the face of the pre-1982 penny. ____ mm 2. Measure the height (thickness) of the penny ___mm 3. Calculate the volume of the penny according the formula for a cylinder. Volume = r2 h, r where =3.14159, r = 0.5 diameter h h = height 4. Repeat for a post-1982 penny. Part E. Calculate Density 1. Using the results from Parts A and C, calculate the densities of pennies. Be sure to include the correct number of significant figures. Density = mass/volume Pre-1982: ________ g/cm3 Post-1982: ________ g/cm3 2. Using the results from Parts B and D, calculate the densities of pennies. Again, watch for the correct number of significant figures. Density = mass/volume Pre-1982: ________ g/cm3 Post-1982: ________ g/cm3 4 Lab IV Write-up: Making Sense of Dense Cents: Making Density Measurements Part A Questions 1. In Part A, why must the boat stay afloat to get an accurate penny mass? 2. How many significant figures are provided in the density of water? 3. In Part A, how many significant figures did you have in your final answer? How strongly do you feel that your masses are accurate? Explain. Part B Questions 4. In Part B, how many significant figures did you have in your final answer? How strongly do you feel that your masses are accurate? Explain. 5. Which penny has the greater mass? 6. Explain the difference between mass and weight. Part D Question 7. In Part D, was there much difference in the volume of the pre-1982 and the post1982 pennies? In Part B, was there much difference in the mass of the pennies? Why is it important that the pennies from year to year are the same size even if they do not have the same mass? Part E Questions: 8. In Part E, compare the results in #1 and #2. Which has more significant figures? Which values do you trust more? Why? 9. Compare your results with those of others in your class, if possible. Is there precision in your class? That is, are your results similar? 10. Look up “accuracy” and “precision”, preferably in a science book. Compare and contrast these terms. How are these distinctions important? 11. In Part E.2., are the densities of the pre-1982 and post-1982 pennies the same? Do you know why they are different? 12. Optional: Have the students find copper and zinc on the periodic table. They are adjacent transition metals, but zinc has a higher atomic number and one more neutron. However, the density of zinc (7.13 g/cm3) is less than that of copper (8.96 g/cm3). Can the students explain why an element with a greater atomic mass might have a lower density? (Hint: Look at a periodic table of atomic radii.) Answers: 1. A completely submerged object will displace a volume equal to its own volume, not its mass. 2. Five. The zero left of the decimal is not significant if there is no digit in the tens place. 3. If a graduated beaker was used, there will be only one significant figure, and it was the uncertain, estimated one. The value determined is likely to be 1 or 0 g.) 4. Answer will depend on the scale used in class. 5. Pre-1982 should weigh about 3.11 g; post-1982 should weigh about 2.5 g. 6. Mass is a measure of the amount of matter in an object. Weight is a measure of the pull of gravity on the mass of an object. 7. The values measures with high precision instruments will have more significant figures, and 5 greater accuracy. A specific type of coin needs to be the same volume, regardless of metallic content, for public use. Consider coin-sorting machines at banks or vending machines. 8. Values measured with low-precision methods will have more variation between students. 9. Answers may vary, but it is possible that there would be precision even among the least accurate measurements. 10. Accuracy implies that the measurement represents the true value. Precision means that there is agreement between repeated measurements. Optimally measurements would be precise and accurate, but these are limited by the methods of measurement. 11. No, the pennies have different densities. The U.S. Mint changed the metal of content from 95% copper/5% zinc&tin to solid zinc (97.5%) inside a thin copper shell (2.5% overall). The density of a post-1982 penny should be closer to the density of zinc. Students may also consider that a penny is not a perfect cylinder (Lincoln’s image, raised rim of the penny, etc.) and that a circulated penny may have some wear and tear, or dirt. 12. The size of an atom is ultimately determined by the arrangement of its electrons. As a trend, atomic radii (i.e. atom sizes) decrease as you move from left to right across the periodic table. Smaller atoms may pack together more tightly which can result in less dense elements. 6 Lab V: Acids and Bases: A study of the pH scale Objectives: To understand the pH scale and the purpose of buffers To understand the terms molarity, molecular mass, acid, and base To understand the use of a logarithmic scale Introduction: Two or more atoms may combine to form molecules. Water is a molecule composed of two atoms of hydrogen and one atom of oxygen. Its molecular formula is written H2O. The molecular formula gives the exact number and types of atoms in the molecule. Molecular formulas are useful in showing how molecules change during a chemical reaction. Water has many properties that are important to life on earth. One of those properties is the ability to dissolve and even change other molecules. For example, when table salt (NaCl) is dissolved, all the atoms dissociate (separate) into positive sodium ions (Na+) and a negative chloride ions (Cl-). Water can even react with itself to cause it to dissociate into ions. See the formula below. H2O H+ + OHA water molecule is separated into a positively charged hydrogen ion (H+)and a negatively charged hydroxide ion (OH-). Therefore some amount of pure water contains a small proportion, or concentration, of H+ ions. Concentration is often measured in molarity (M, or moles per Liter). If water dissolves molecules that are acids or bases, the concentration of H+ ions will change. This is because acids add H+ to the solution; bases remove H+ ions from the solution. The pH scale helps us to visualize the concentration of H+ ions that are in the solution. Because the concentration of H+ ions can vary by orders of magnitude (powers of 10), the pH scale is a logarithmic scale. A logarithm is an exponent of a base number, such as 10. So moving to the left on the pH scale by just one unit represents 10fold higher concentration of H+ ions! Acids (and bases) can be strong or weak, depending on how well the molecules dissociate from their H+ ions. Weak acids and bases are found in the middle of the pH scale and are helpful in laboratories and especially the human body because weak acids and weak bases can prevent fast and large changes in pH. Therefore, weak acids and bases are sometimes called pH buffers. Supplies: Baking soda (called sodium bicarbonate or NaHCO3) White distilled vinegar (typically 5% acetic acid, C2H4O2) pH paper with a broad range from pH 1 to pH 14 Distilled water Plastic transfer pipettes or medicine droppers Tweezers Graduated cylinders and beakers Metric kitchen or jewelry scale Picture of the pH scale Teacher Prep: 7 Just before class, prepare a 6.4% solution of sodium bicarbonate (6.4 g baking soda per 100 mL distilled water at room temperature). Each student or student pair will need 25 mL. Alternatively, students may make their own solution. The solution should not be made earlier than the night before because sodium bicarbonate solutions break down. Titration Procedure: - A titration can help one measure the amount of the acid or base. 1. Measure 10 mL of vinegar in a graduated cylinder. Transfer the vinegar to a small beaker or cup. 2. Obtain or make 25 milliliters of a concentrated solution of baking soda (NaHCO3). 3. Use a small piece of pH paper (~1cm long) to measure the pH of the vinegar. Record the pH here ____ and in the table below. 4. Test the pH of the baking soda solution. Record the pH ____. 5. Add 1 mL baking soda solution to the cup of vinegar at a time. Be sure to swirl the cup to mix thoroughly. Test the pH with a small piece of pH paper. Record the volume added and new pH value in the table below. 6. Make observations. Pay attention to the formation of bubbles. Mark with an asterisk (*) the pH values when you observe bubbles. 7. Repeat 5-6 until the pH is 8 or higher. 8. Graph your results as pH vs. sodium bicarbonate in milliliters. Volume NaHCO3 added now (mL) 0 1 1 1 Total NaHCO3 added (mL) 0 1 2 3 pH 8 Your students’ graphs may look something like this. 9 10 Lab V Write-up: Acids and Bases: A study of the pH scale Questions (Students may need to use the internet or an introductory chemistry or physical science book to help with some of the answers.) 1. Describe how table salt (NaCl) changes when it is dissolved in water, and balance the following chemical equation that shows what happens. If the answer is an ion, remember to include the electrical charge. NaCl Na+ + ____ 2. Describe your observations during the titration. At what pH did you observe bubbles forming? At what pH did they stop forming? What molecule(s) are the bubbles? Why do they stop forming near the end of the titration? 3. An acid may be defined simply as a “proton donor”. Explain how acetic acid in vinegar is a proton donor. 4. Explain how the pH scale is a logarithmic scale. 5. Hydrochloric acid has the chemical formula HCl. It is a strong acid that completely dissociates into its ions in water. Balance the following chemical equation. HCl ____ + Cl6. Sodium bicarbonate is a weak base. How is a base different from an acid? 7. Optional. Read the following and answer the questions below. The Hindenburg Disaster occurred on May 6, 1937. The Hindenburg was a German passenger airship that was inflated with hydrogen, a light and highly flammable gas. Tragically, the hydrogen gas in the airship was ignited into flames. The following is a balanced equation of a hydrogen combustion reaction. 2H2 + O2 2H2O a. What are the reactants of this equation? The products? b. How many atoms of hydrogen are in the hydrogen molecule? The oxygen molecule? c. The water molecule is composed of two hydrogen atoms and one oxygen atom. In the equation above, what does the coefficient “2” in front of “H2O” mean? Answers: 1: Salt molecules dissociate into their respective ions when dissolving in water. NaCl Na+ + Cl2. Bubbles form because of a reaction of the H+ ions from the vinegar with the bicarbonate ion (HCO3-). The bicarbonate ion with the hydrogen ion becomes carbonic acid (H2CO3), which is unstable and quickly breaks down into water (H2O) and carbon dioxide (CO2). The latter is a gas. At the end of the titration (pH = 6.0 and above), the H+ ions from the acetic acid have been used up, so the bubble-forming reaction stops. 3. Acetic acid dissociates into H+ ions and acetate ions (C2H3O2-) in water. Thus, it increases the concentration of H+ ions in a water solution. 11 Advanced students might add something like this: Most hydrogen atoms have only one proton and one electron. When the hydrogen ion dissociates from the acid molecule, it leaves its lone electron on the negative ion. So only the H+ ion is a proton only. 4. The concentration of H+ ions can vary by orders of magnitude (powers of 10). The pH scale is a logarithmic scale, to simplify the writing of long numbers. A logarithm is an exponent of a base number, such as 10. So moving to the left on the pH scale by just one unit represents 10-fold higher concentration of H+ ions. 5. H+ 6. A base is a proton acceptor, that is, it decreases the concentration of H+ ions in water. 7a. The reactants are H2 and O2. 7b. There are two atoms of hydrogen in a hydrogen molecule and two atoms of oxygen in one oxygen molecule. Explanation: Some elements are so reactive that they cannot exist as unbonded atoms. Pure hydrogen or oxygen must exist as diatomic molecules. 7c. The coefficient shows how many of the particular molecule are involved in the reaction. Two molecules of hydrogen react with one molecule of oxygen to yield two molecules of water. 12 Lab VI. Using Tums to tackle the Gas Laws Objective: To use the equations of the gas laws to measure a chemical reaction To set up a gas trap for a chemical reaction Solid, liquid, and gas are the three states of matter typically observed on earth. Unlike the other two states of matter, gases have neither a fixed volume (liquids, solids) nor a fixed shape (solids). Here, we will discuss three of the laws that dictate the properties of gases: Boyle’s Law, Charles’s Law, and the Ideal Gas Law. Boyle’s Law (17th century, Robert Boyle) states that the pressure and volume of a gas are inversely proportional, when the temperature is held constant. Charles’s Law (18th century, Jacques Charles) states that the volume of a gas held at a constant pressure is proportional to its absolute temperature on the Kelvin scale. (The Kelvin scale begins at absolute zero, which is equivalent to -273 Celsius.) Boyle’s Law: P1V1 = P2V2 Charles’s Law: V1 = V2 T1 T2 The first two laws are simply written but more difficult to employ because (1) of the need to hold constant pressure or temperature (2) initial and final measurements typically must be made. The Ideal Gas Law is derived from the first two laws and another (Avogadro’s Law) which states that the number of moles of the gas is proportional to the volume. A mole of a substance is equivalent to Avogadro’s number, 6.02 1023. Avogadro’s number: 1 mole = 6.02 1023 molecules, atoms, or ions Ideal Gas Law: PV = nRT where P is pressure in atm, V is volume in L, n is the number of moles, R is a constant, 0.08206 L-atm/mol-K T is temperature, in Kelvin The Ideal Gas Law works well for “ideal gases”, which includes this experiment. The Ideal Gas Law can be used to calculate how many moles of something is generated during a chemical reaction while measuring pressure, temperature, and volume only once. Here, we will react calcium carbonate (CaCO3) in a Tums tablet with the acid in vinegar, and measure the amount of carbon dioxide gas produced. The overall chemical reaction is written: CaCO3 + 2H+ CO2 + Ca2+ + H2O 13 Supplies: • Tums tablets • White distilled vinegar (5% acetic acid) • Graduated cylinders and beakers (or clear cups) • Squares of wax paper (to use as weigh paper on the scale) • 3/16" diameter, or smaller, vinyl tubing • 1-L or 16-oz plastic soda bottles (2 per student or student pair) • Cap to soda bottles with drilled holes (1 per experiment; holes should give a snug, airtight fit to the tubing) • Erlenmeyer flasks • Rubber or cork stoppers with tube-sized holes drilled through them (stoppers must fit the flasks; holes must snugly fit tubing) • Rolling pin (or hammer) and cutting board • Permanent marker • Kitchen or jewelry scale • Barometer (or at least instantaneous access to barometric pressure readings in your area, e.g. weather.com) • Air thermometer (or immediate access to local or indoor temperature readings) • Funnels (optional) Teacher Prep and Tips: This is a good experiment for students to do in pairs. Some steps will go better with more than two hands. In Part A, students will determine how much vinegar must be added in Part B to release all the CO2 that is possible. In Part B, students will make the gas trap according to your instructions. You may use either 1) an Erlenmeyer flask, a cork or rubber stopper, a beaker, and a plastic soda bottle without a cap; or 2) two soda bottles and one airtight cap, and a beaker. Students will pulverize a Tums tablet, place the powder into a flask or bottle, quickly add the full volume of vinegar, and plug up the top with a cap, rubber stopper, or cork (whatever you have for them). A narrow tube leads through the plug and into another bottle, filled with water, and turned upside-down in a beaker with water. The plug (or bottle cap) must have a drilled hole that exactly fits the tubing. The upside-down bottle must not have a cap. The students must mark the water level in the bottle before adding the vinegar to the flask. As gas is made in the flask, air bubbles from the tube will displace the water in the upside-down bottle. As air fills the bottle, water may overflow the beaker during the experiment, you may want students to set it up in a secondary container, like an old pan. 14 Students must mark the final water level in the bottle before disassembling the trap. Then they can use the two marks to determine the volume that was displaced. Procedure: Part A: Tums Titration 1. Read the ingredients label on the Tums. Look for the mass of calcium carbonate (CaCO3) in each tablet. If the mass is in milligrams, convert the mass to grams by dividing by 1000. Record the mass. _____ g 2. Weigh a single Tums tablet on the scale. Use wax paper to keep the scale tidy, but remember to subtract its weight. Record the mass of the tablet here. ______ g 3. Divide the mass of CaCO3 by the total mass of the tablet and record here. _____ 4. Pulverize a single Tums tablet on a cutting board by pressing with a rolling pin. (Or use any effective method.) 5. Fold a square of wax paper in half twice with perpendicular folds. If your scale has a “Tare” button, then place the paper on the scale and press “Tare”. This will allow your scale to ignore the mass of the paper. If the scale does not “Tare”, then weigh the wax paper so that you may subtract its mass later. 6. Transfer the Tums powder to the wax paper. The folds will help keep the powder from spilling. Record the mass of the powder here. ______ g 7. Multiply the mass of the powder by the fraction determined in #3. _____ g This is the mass of CaCO3 that should be in the powder you recovered. 8. Transfer all the powder on your wax paper to a large (150) mL beaker or flask. 9. Measure 100 mL vinegar in a graduated cylinder. Add the vinegar in 10 mL increments, swirling between additions. Bubbles should form. 10. Continue adding acetic acid until bubbles no longer form. How much vinegar did you need to add? _____ mL 11. Divide the volume of vinegar added by the mass of the powder from step 6. Record here. _______ mL/g Part B: Setting up a gas trap In this section, you will add an excess amount of vinegar to another pulverized Tums and capture the bubbles formed. 1. Pulverize another Tums tablet and weigh the mass of the powder on a folded piece of wax paper. Remember to subtract or tare the mass of the wax paper. _____ g 2. Transfer the Tums powder to a dry flask (or bottle). 3. Feed the tube through the hole in the stopper. (If you are using a plastic bottle instead of a flask, then put the tube through the drilled hole in the bottle cap.) The tube should be airtight in the stopper. Set this aside. 4. Set up the gas trap as instructed below. a. Calculate the amount of vinegar to add by this method: multiply your answer in Part A, step 11 (mL/g) by the mass of powder for Part B, step 1. Multiply that volume by 1.1 and enter the result here. _____ mL b. Note: The volume you calculate in Step 4a has added 10% more vinegar than you should need to ensure that the maximum amount of CO2 is produced. In other words, the vinegar is added in excess. c. Fill a second bottle with tap water. 15 d. To a beaker, add only enough water that will allow you to turn the water-filled bottle upside-down in it without losing much water from the bottle. Place this beaker in a secondary container. e. Place the free end of the tubing into the water-filled bottle. Quickly, turn the bottle upside down into the beaker. Keep the stopper end of the tube up so that the tube does not fill with water. f. Important: use a permanent marker to mark the water level on the upside-down bottle. This is the initial volume (Vi) g. Measure out the vinegar you calculated in Step 4a. Read the next step carefully all the way before starting the reaction. h. Working quickly, pour the vinegar into the flask with the Tums powder and tightly seal with the stopper (or bottle cap). i. Gently swirl the flask until bubbles stop forming. A gas will displace the water in the upside-down bottle. Watch that the water in the beaker does not overflow. 5. When no more gas is being formed, mark the final volume (Vf) of water in the gastrap bottle. 6. Disassemble the gas trap. Fill the gas-trap bottle to the Vi mark with water. Pour this water to a graduated cylinder. Record the Vi. ____mL 7. Repeat step 6 to measure the Vf. Record the Vf. ____mL 8. Subtract the Vi from the Vf. This is the change in volume (V). Record the V ____mL 9. Use a barometer to record the atmospheric pressure. Be sure to include the units of your barometer. “Atmospheres” (atm) is the preferred unit. _____________ 10. Use a thermometer to measure the air temperature. Be sure to include the units of your thermometer. “Kelvin” (K) is the preferred unit. _____________ 16 Lab VI Write-up. Using Tums to tackle the Gas Laws Questions Part A 1. How much of the Tums tablet is “inactive ingredients”? What are some of these ingredients? Why are they in tablet? 2. What ion is reacting with the Tums? Which part of the human body has a high concentration of this ion? Part B 1. Convert all your measurements (if necessary) for use in the Ideal Gas Law equation. Show your work. Volume. V (mL) 1L = V in L 1000mL Pressure. P (mm mercury) 1 atm = Pressure in atm mm Hg Temperature, From Fahrenheit to Kelvin T(K) = (T(°F) + 459.67) × 5/9 Temperature, from Celsius to Kelvin T(K) = (T(°C) + 273.15) 2. Use the Ideal Gas Law to find “n”. PV=nRT, rearrange to: n=PV RT Where R = 0.082 atm*L/mole*K n = ___________ moles CO2 3. The theoretical yield of a reaction tells us how much product (CO2) you should get if the experiment went perfectly. Follow the steps below to calculate the theoretical yield of CO2. a. Use a periodic table to find the molar mass of CaCO3. To do that, find the atomic mass of each atom in the molecule. For oxygen, you must first multiply its mass by three since there are three oxygen atoms in the molecule. Ca =_______ g/mol C =_______ g/mol O3 = _______ g/mol Sum of values: ____________ g/mol CaCO3 b. What mass of CaCO3 was reacted in Part B? (Hint: # g powder, Part B step1 #g CaCO3 per tablet = #g CaCO3) #g of a whole tablet 17 c. How many moles of CaCO3 were reacted? (Hint: Divide the answer from 3b by the answer from 3a.) ___________ mol CaCO3 d. Now, one molecule of CO2 is made from every molecule of CaCO3 CaCO3 + 2H+ CO2 + Ca2+ + H2O Therefore, the value in #2 should equal the result in 3c. Is that what happened? Why not? Answers Part A 1. In an extra strength Tums, there are 1000 mg (1g) calcium carbonate, but the whole tablet is 2.6 grams. The main ingredient is sucrose, or table sugar. 2. H+ reacts with the calcium carbonate. The stomach has a low pH and a high H+ concentration. Part B 1-2. Students should do the math carefully as the Ideal gas law will not work if the correct units are not used. 3a. Ca =40.08 g/mol C =12.01 g/mol O3 = 16x3=48.00 g/mol Sum of values: 100.09 g/mol CaCO3 3b. If 2.4 g powder reacted, then 2.4 g x 1 g/2.6g = 0.92g 3c. Example: 0.92g 100.09 g/mol = 9.2 x 10-3 mol CaCO3 3d. Experiments are not perfect. Maybe there wasn’t exactly 1000 mg calcium carbonate per tablet, maybe not all the carbonate reacted with vinegar, maybe some gas leaked, maybe students measured volume incorrectly, etc. 18