American Journal of Neuroradiology
advertisement

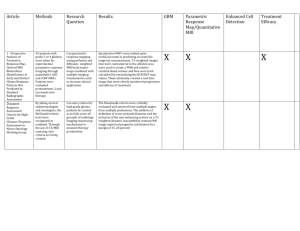
Radiological features of cerebellar glioblastoma multiforme Dept. of Radiology and Neurosurgery Ehime University School of Medicine K. Kikuchi, Y. Hiratsuka, S. Ohue, T. Mochizuki Introduction Glioblastoma (GBM) is the most common type of malignant CNS tumor in adults; however, the prevalence of GBM that arises in the cerebellum is extremely low. Malignant glioma is rarely considered in the preoperative differential diagnosis of cerebellar tumors. Although the possibility of cerebellar glioma is noted primary consideration is given to metastatic brain tumor, hemangioblastoma, and primary CNS lymphoma (PCNSL). Propose The purpose of this study was to demonstrate the radiological features of cerebellar GBMs, including CT, MRI, and FDG - and MET - PET findings. Materials and Methods We retrospectively reviewed patients with glioma who underwent craniotomy or stereotactic biopsy at our hospital from March 2003 to June 2014. The inclusion criterion was pathologically proven cerebellar GBM in an adult patient. Patients with supratentrial GBM were excluded. Seven patients (six men and one woman: mean age: 56 years, range: 18 - 73 years) satisfied these criteria and were included in the study. Materials and Methods We reviewed the medical records and radiological images. No-contrast CT (NCCT), MRI and PET. NCCT and MRI (T2WI, FLAIR, T1WI and Gd enhanced T1WI) were performed in all patients. FDG- and MET-PET examinations were performed in three patients. Materials and Methods MRI 1.5-T (n = 3) or 3-T system (n = 4). T2WI by fast SE T1WI by SE or fast IR Contrast medium at 0.1 mmol/kg body weight. PET MET: A 21 minute static PET scan was begun 20 minutes after MET injection (5 MBq/kg body weight) FDG: After MET acquisition, FDG (3.5 MBq/kg body weight) was injected. Rest for 90 minutes after injection, emission data was acquired for 20 minutes. Patients Summary No. Age/Sex Symptom 1 33/M Headache 2 71/F None 3 70/M Cerebellar ataxia 4 71/M None 5 56/M Dizziness, Cerebellar ataxia 6 18/M Headache, Vomitting, Cerebellar ataxia, Nystagmus 7 73/M Dizzienss, Vomitting Image Findings: CT & MRI No. CT MRI 1 iso~low 2 low 3 not evident 4 iso irregular ring enhancement 5 slightly high homogenous enhancement 6 low, hemorrhage 7 low Lermitte-Duclos like irregular ring enhancement multiple patchy enhancement hemorrhage, partially enhancement homogenous enhancement with necrosis SUVmax on PET No. FDG SUVmax MET SUVmax 3 12.1 9.3 4 8.8 5.3 5 22.2 10.1 Case 1: 33/M Case 1 had a “tiger-striped” appearance with faint contrast enhancement on MRI and was accordingly diagnosed preoperatively as Lhermitte-Duclos disease. However, the confirmed pathological diagnosis was cerebellar GMB. To our knowledge, this is the first report of a case of cerebellar GBM with a Lhermitte-Duclos disease like “tigerstriped” appearance. The reasons for these imaging findings are unknown, and the preoperative differential diagnosis was therefore extremely difficult. Case 2: 71/F The appearances of GBM vary widely, although the most important unifying characteristic, which is recognized during both radiologic and gross pathologic examinations, is the presence of hemorrhage or necrosis. The primary cellular component of the tumor exhibits variable attenuation on unenhanced CT and depending on tumor cellularity, hypointensity on T1WI. The presence of necrosis is clearly demonstrated as a ring enhancement on Gd-enhanced T1WI and serves as a key indicator of GBM diagnosis. Case 6: 18/M In this case, intra-tumoral hemorrhage was depicted strong attenuation on unenhanced CT. On T2WI, a hyperintense rim surrounding a hypointense area, which was also suggestive of hemorrhage, was exhibited. A ring enhancement was also shown surrounding the hemorrhage. In our patient series, an irregular ring enhancement finding on MRI was evident in three cases, and a solid tumor with necrosis was evident in one case. These four cases could be prospectively diagnosed as cerebellar GBM. Case 3: 70/M FGD MET Cases 3 and 5 exhibited strongly increased FDG and MET uptake, and PCNSL was assumed as a preoperative diagnosis. In these cases, the MET uptake area was larger than the Gd enhancement area. Sasaki et al. reported a larger area of MET uptake than the areas of FDG uptake and Gd enhancement in astrocytic tumors. In contrast, Ogawa et al. reported that the area of increased MET accumulation was larger than those of the enhancing lesions observed on CT or MR images from patients with PCNSL. Case 5: 56/M FDG MET FDG-PET has been reported as a useful tool for differentiating PCNSL from high-grade glioma. Makino et al. and Okada et al. reported that a FDG SUVmax of ≥12 indicated PCNSL, whereas a value <12 suggested GBM; however cases 3 and 5 of our GBM patients had FDG SUVmax values >12. Discussions GBM is the most common type of primary CNS tumor and is located most frequently in the cerebral hemispheres, with a peak age of onset during the sixth or seventh decade of life. According to previous reports, cases of GBM in the cerebellum are extremely rare. The theory in which cerebellar GBM originates from embrionarycells was altered following the proposed anaplasic progression of mature cerebellar astrocytic cells. Therefore, there is no apparent explanation for the rarity of the infratentorial compartment as a site of GBM origin. Discussions MRI is the diagnostic method of choice for a suspected intracranial tumor. Nevertheless, cerebellar GBM can be easily misdiagnosed, even after performing contrast-enhanced high-resolution MRI because the imaging findings are often unspecific. A discrepancy between the MET uptake area and lesion enhancement area on MRI suggests a diagnosis of glioma or PCNSL. FDG SUVmax has been reported as a useful tool for differentiating PCNSL from high-grade glioma. In our patients series, however, the differential diagnoses were difficult despite referring SUV max values. Conclusion We reviewed the radiological features of seven cases of cerebellar GBM. Some of these cerebellar GBM cases did not exhibit common CT, MRI, and PET findings. Therefore, the consideration of CT, MRI, and PET findings in the differential diagnosis of cerebellar GBM remains challenging. References Gopalakrishnan CV, Dhakoji A, Nair S, et al. J Clin Neurosci 2012;19:1684-1688 Grahovac G, Tomac D, Lambasa S, et al. Acta Neurochir (Wien) 2009;151:653-657 Gupta V, Goyal A, Sinha S, et al. J Neurosurg Sci 2003;47:157-164 Kuroiwa T, Numaguchi Y, Rothman MI, et al. American Journal of Neuroradiology 1995;16:583-589 Mattos JP, Marenco HA, Campos JM, et al. Arq Neuropsiquiatr 2006;64:132-135 Akimoto J, Fukami S, Tsutsumi M, et al. Brain Tumor Pathol 2009;26:59-68 Chiofalo M, Cappabianca P, Del Basso De Caro M, et al. Journal of Neuro-Oncology 2007;82:183-185 Thomas B, Krishnamoorthy T, Radhakrishnan VV, et al. Neuroradiology 2007;49:733-738 Sasaki M, Kuwabara Y, Yoshida T, et al. Eur J Nucl Med 1998;25:1261-1269 Ogawa T, Kanno I, Hatazawa J, et al. Radiographics 1994;14:101-110 Thank you for your attention