Biology 121 Hybrid Week 4
advertisement

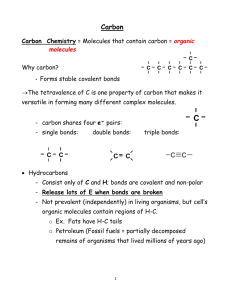
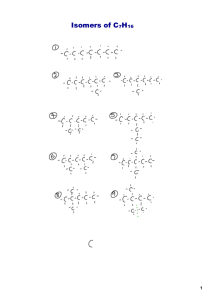
Biology 121 Lectures 2.1 & 2.2 Organic Molecules Functional Groups Chemical Reactions Organic? That means ‘healthy’, right? • The term “organic” has developed a vernacular meaning in our language, causing many people to associate organic with the terms “healthy” and “natural”. Organic? Aren’t those kind of dangerous? • In chemistry, the term organic maintains its original meaning. As you’ll find, in the scientific context, some organics are healthy, but many are synthesized in the lab, so they aren’t “natural” – and they aren’t necessarily healthy! Organic? What does that mean? • Organic chemistry is the study of organic molecules. What organic molecules all have in common is… • Carbon! • More specifically, C and H. Organic means C and H • So CO2 is generally not considered an organic molecule, but CH4 is. • CH4 = organic • CO2 = inorganic • C6H12O6 = organic • H2O = inorganic Categorizing Organic Molecules • The compounds in the human body are organic -- we are a carbon-based organism. • Organic compounds are the building blocks of life. • There are 4 major categories: – Carbohydrates – Lipids – Proteins – Nucleic acids Categorizing Organic Molecules • Keep in mind the nature of C bonds. C-C and C-H bonds are very nonpolar. • H2O is a very polar molecule, and our bodies are 60-70% water. We are a very polar environment, and simple hydrocarbons would not fare well in our tissues! • Biologically important carbon molecules need other atoms like O and N that are more electronegative to add polarity to the molecules. Why Carbon? • C has characteristics which make it a good choice for this very central role in biology. Nature of Carbon Bonds - Variety 1. C has 4 valence e– C can form 4 covalent bonds to satisfy a full valence shell. – An example of a satisfactory arrangement is CH4, commonly known as methane. Methane is one of the simplest organic compounds. – These 4 covalent bonds yield lots and lots of possible combinations Nature of Carbon Bonds - Variety 2. C’s 4 covalent bonds are very strong, but are still breakable by cells – Normal biological methods can break and reform these bonds 3. C bonds allow rotation to form lots of shapes and conformations Nature of Carbon Bonds - Variety 4. C can form single, double or triple bonds H H H H H C C C C H H H H H Butane • Here are 3 similar molecules, but C is single, double or triple-bonded. H H H C C C C H H H H H Butene H H H C C C C H H H Butyne Categorizing Organic Molecules • For any given pair of ions, there was ONE possible arrangement to make an ionic compound. • In covalent bonds, any unpaired e- in the carbon shell can bond with ANY other atom with unpaired e- . The possibilities are endless, so there are many more types of organic molecules, and they can become incredibly large and complex, even when there are only C and H bonding. Categorizing Organic Molecules • Simple organic molecules contain just C and H = hydrocarbons • But more complex organic molecules contain C and H, but also O, S, P, and N and other elements • With all this complexity, we’re going to need some sort of system to categorize the myriad organic compounds into recognizable groups. Categorizing Organic Molecules 1. Start simple. Study the bonding patterns of compounds that consist of ONLY C and H. 2. Add on polarity with other atoms, such as O, onto our C framework, noting how the compound changes. – Functional groups 3. With that system in place, we’ll go on to consider carbohydrates, lipids, proteins and nucleic acids IUPAC Nomenclature • As we scrutinize and categorize organic compounds, we’ll name them based on their bonding. Naming is also referred to as “nomenclature”. • Naming compounds allows us to develop a common language, so that we can discuss compounds easily. • It’s helpful if we all use the same naming system. IUPAC is the chemistry organization responsible for setting naming rules. Even in biology, we follow their guidelines. Start With the Simplest Carbons • To begin, we’ll consider molecules that ONLY contain C and H. – Hydrocarbons • First, we’ll consider only hydrocarbons that have all single bonds. – Alkanes Start With the Simplest Carbons • Start with 8 alkanes, listing C number, name, and structure. • Each compound has an ”ane” ending, because they are alkanes. • The prefix for each compound is assigned based on its C number. – 1 C is meth– 8 Cs is oct- • have formula CnH2n+2 “Straight” carbon chains • We call hydrocarbons that have all the Cs arranged linearly (in a straight line) “straight chain” hydrocarbons. • As the ball and stick models to the right indicate, they actually aren’t “straight” at all, that’s because of the tetrahedral shape of the individual C atoms. They aren’t straight for the same reason water isn’t straight. • Flexible, can bend and rotate Double Bond Alkene • Cs can bond through more complex patterns than just single bonds, forming double and even triple bonds with one another. • If there is at least one double bond in a hydrocarbon, we call it an alkene. Its prefix stays the same, but the ending is now –ene. • All have formula CnH2n • Must lose 2 H to form a double bond • Double bond is more rigid, no rotating around double bond ethane ethene Triple Bond Alkyne • If there is at least one triple bond in a hydrocarbon, we call it an alkyne. Its prefix stays the same, but the ending is now –yne. • All have formula CnH2n-2 • Lose 2 more H to form a triple bond. • Triple bond is also rigid, no rotating around triple bond ethane ethene ethyne Double and Triple Bonds – fewer Hs • Ethane = C2H6 • Add a double bond, remove two Hs. Ethene = C2H4. • Add a triple bond, remove two more Hs Ethyne = C2H2 ethane ethene ethyne Double and Triple Bonds • C-C single bonds in alkanes are movable. The Cs can spin with respect to one another, causing the Hs to spiral like spokes on a wheel. • In ethene and ethyne the C-C double and triple bonds cannot move, so the Hs are stuck in one place. ethane ethene ethyne Start With the Simplest Carbons • Learning Goal: Be able to name molecules based on the IUPAC guidelines for C1 through C8 compounds Name That Molecule Name That Molecule 3 C, one triple bond = propyne C3H4 5 C, all single bonds = pentane C5H12 6 C, all single bonds = hexane C6H14 8 C, one double bond = octene C8H16 Hydrocarbons can have many structures 1. Straight chain – All Cs bonded together in one line (even if bent due to rotation around bonds) – Can be very long (25+ Cs) H H H H H H H H C C C C C C C C C H H H H H H H H H H Hydrocarbons can have many structures 2. Branched chain – Main chain has one or more Cs attached as a side chain – Named according to the longest continuous chain • Branched propane • Branched heptane • Branched decene H H H H H H H H H H H C C C C C C C C C C H H H H H H H H H C H H C H H Naming Branched Chains • The trick for branches, is to name the molecule based on the number of the longest C chain, and treat the branch as an “accessory”. So the bottom compound is a “heptane”, with a 3C branch off of the middle C. Naming Branched Chains • The real name of this compound is 4-propyl-heptane. We will not be covering IUPAC rules of naming such complicated molecules, you simply need to know this as a branched heptane for now. 3. Ring Structures • It is also possible for a chain of C to bend around so that the end C bonds with the first C, forming a ring. – Rings can be as small as 3 C, but 5 and 6 C rings are the common sizes we’ll see in biology. • To name such compounds, add “cyclo” to the front. So a 6C ring with all single bonds is called cyclohexane. • Lose 2 H to make the C-C bond to close the ring. • Some rotation around bonds, but less flexible than chains. Rings • Notice that when a hydrocarbon turns into a ring, you need to remove two Hs to accommodate the extra C-C bond - just like adding a double bond. • Notice, also, that the ring structure holds the Hs rather rigidly in place, they aren’t free to spiral anymore - just like adding a double bond. • Can have single, double, triple bonds, branches, more rings, etc. Benzene – a special ring • Benzene, C6H6 is a very special molecule in chemistry. It has an unusual bonding arrangement which puzzled chemists for years. A rather simple representation (which suits us just fine) is to think of benzene as a 6-cabon ring with alternating single and double bonds. • Benzene is not found in the human body (it is actually a carcinogen), but there are many derivatives of benzene -- compounds which contain a benzene ring -- found in nature. They are called “aromatics” because they have pleasant aromas, you’d find them in rose oil, for example. Chain, Branched and Ring Hydrocarbons • Learning Goal: For carbon chains between 1 and 8 carbons long, including straight-chain, branched and rings, be able to name molecules based on the IUPAC guidelines What’s missing from these structures? • Now that we’re such good chemists, we’re going to need to understand some common shortcuts – Leave Hs off, assume maximum Hs whenever they’re omitted – C at every corner unless something else is indicated cyclopentane heptane More Name That Molecule! 4 C, ring, only single bonds = cyclobutane 2 C, straight chain, only single bonds = ethane 5 C, branched chain, only single bonds = branched butane 5 C, straight chain, only single bonds = pentane More Name That Molecule! 4 C, ring, only single bonds = cyclobutane 2 C, straight chain, only single bonds = ethane 4 C, branched chain, only single bonds = branched butane 5 C, straight chain, only single bonds = pentane More Name That Molecule! 7 C, branched chain, only single bonds = branched heptane 8 C, straight chain, double bond = octene 5 C, ring, single bonds = cyclopentane 4 C, straight chain, double bond = butene More Name That Molecule! 7 C, branched chain, only single bonds = branched heptane 8 C, straight chain, double bond = octene 5 C, ring, single bonds = cyclopentane 4 C, straight chain, double bond = butene Isomers – same formula, different structure • Because C is such a ‘flexible’ atom it can bond in many different arrangements. • Even for simple hydrocarbons, you can have two different molecules with the same molecular formula – different spatial arrangements in space • For example, C4H10 can be a straight chain OR branched. • If we needed to distinguish which of these two isomers we wanted, we would need to expand the molecular formula, as in the figures to the right. • Important because different structures have different functions Isomers – same formula, different structure 3 types of isomers – 1. Structural – same formula, different bond arrangement 2. Geometric – same formula and bonds, but held in different positions – Requires a double bond 3. Enantiomers – same formula, same bonds, but held in different arrangement around a central C. – Requires 4 different groups on C (chiral) – Non-superimposable mirror image How Many Structural Isomers are Possible for the Alkanes? No. of C Atoms Molecular Formula 1-3 Possible Isomers 1 4 C4H10 2 5 C5H12 3 6 C6H14 5 7 C7H16 9 8 C8H18 18 No memorization necessary! Drawing Structural Isomers • Different isomers often have different names – don’t worry about this in Biol 121 • Learning Goal: For a given molecular formula of a hydrocarbon, be able to draw a stated number of different structural isomers Sample Problems • Draw 2 structural isomers of C4H10. 1. Any rings, double or triple bonds? – Compare no. of Cs and Hs 2. Draw straight chain 3. Reduce chain by 1C and add 1C branch(es) – Move branch around first half of chain 4. Reduce chain by 2C and add 2 1C branch(es) – Move branch around first half of chain 5. Reduce chain by 2C and add 2C branch(es) – Move branch around first half of chain 6. Draw rings, rings with branches, etc. Sample Problems • Draw 2 structural isomers of C4H10. H H H H H C C C C H H H H H H HCH H H HC C CH H H H Sample Problems • Draw 6 structural isomers of C7H16. • Draw 4 structural isomers of C7H14. Two More Types of Isomers • In addition to the structural isomers we have already discussed, there are two more special situations where we need to be able to distinguish between similar, but different, molecules. – Geometric isomers occur around double bonds – Enantiomers occur every time a carbon has 4 different atoms attached to it 2. Geometric Isomers • A special type of situation exists around double bonds. Remember that single bonds can rotate like wheel spokes. But double bonds are held firmly in space. • In the example molecule on this page, dichloro-ethene, the green chlorine atoms can either be attached to the same sides of the double bond, or to opposite sides. • When atoms are on the same side, they are cis. When they are on different sides, they are trans. These are different geometric isomers, because the chlorines cannot rotate around the double bond from one side to another. • For di-chloro-ethane, the chlorine atoms are free to rotate from one side to another, there are no geometric isomers to worry about for that molecule. 2. Geometric Isomers • Need to have a double bond so no rotation • Groups held in different positions • Each C must have 2 different groups attached. • If one C has 2 of same group, not isomers H3 C H C H H3C CH3 C C H H Not geometric isomers C CH3 3. Enantiomers • Enantiomers have same bonds, but 4 different groups arranged differently around a central C. • C in middle, hold one atom at top & rotate to make match. No match enantiomers 3. Enantiomers • If can rotate to match not enantiomers • If no possible match enantiomers • If enantiomers, central C is a chiral C. not enantiomers – 4 different groups attached to C – Black sphere is a chiral C in bottom enantiomer pair enantiomers Biology 121 Lectures 2.1 & 2.2 Organic Molecules Functional Groups Chemical Reactions Functional Groups add Flavor • Remember that hydrocarbons (organic molecules with only Cs and Hs) are – Nonpolar covalent and are not soluble in H2O – Undergo combustion, but are otherwise fairly unreactive. • In contrast, the biologically significant organic molecules we need to study are generally – polar covalent and – more reactive. – contain O, N and other atoms. Functional Groups add Flavor • Functional group = atom or group of atoms added to hydrocarbons that change their properties • Each functional group – Name – Formula – Function • Good charts and descriptions in book • R always indicates “rest of the molecule,” anything No functional group • Hydrocarbon only – C-H – Non-polar, do not interact with H2O – Interact weakly with each other • Hydrocarbon branches – R-CH3 – R-CH2-CH2 methyl group ethyl group H H │ │ H – C – C – H │ │ H H ethane 5 ways to add O 1. Hydroxyl R-OH – Polar bond, makes molecule polar and hydrophilic – Family = alcohols – Increases cohesion by allowing interaction of molecules (ethanol is liquid at RT whearas ethane is a gas) H H │ │ H – C – C – OH │ │ H H ethyl alcohol, ethanol 5 ways to add O 2. Oxyl R-O-R – formed when molecules w/ -OH join together – Family = ethers – provides place to break larger molecules apart CH3CH2-O-CH2CH3 diethyl ether 5 ways to add O 3. Carbonyl R=O – polar bond, make molecule hydrophilic – 2 kinds of carbonyl groups a. Aldehyde • carbonyl at the end of the molecule • Family = Aldehydes b. Ketone • carbonyl in the middle of the molecule • Family = ketones O ║ R–C–H O ║ R–C–R O ║ H – C – H formaldehyde H O H │ ║ │ H–C–C–C–H │ │ H H acetone 5 ways to add O 4. Carboxyl R-COOH – very polar, two O have very strong pull, can take electron from H, creates ions – Family = carboxylic acids – R – COO- + H+ = weakly acidic – important in amino acids to make proteins O ║ R – C – OH H O │ ║ H – C – C – OH │ H acetic acid 5 ways to add O 5. Ester R-COO-R – formed when molecule w/ -OH joins carboxyl from H, creating ions: R – COO- + H+ – Family = esters – can donate H+ so weakly acidic (- charge) – important in amino acids to make proteins O ║ R – C – OR O ║ CH3–C–O–CH3 Other Atoms - Add an N 6. Amino R-NH2 – can accept a H+ to become R – NH3+ – Family = amines – weakly basic (+ charged) – important in amino acids and nucleic acids H │ H – C – NH2 │ H Other Atoms - Add a P 7. Phosphate R-H2PO3 – very polar, strong pull of O can take electrons from one or both H, creating ions R – PO42- + 2H+ – Family = organic phosphates – weakly acidic (- charge) – found in nucleic acids O ║ R – P – OH │ OH Other Atoms - Add an S 8. Sulfhydryl R-SH – polar due to electronegativity of S – Family = thiols – found in amino acid cysteine SH │ H – C – │ H NH2 │ C – │ H O ║ C – OH cysteine (amino acid) Other Atoms - Add 2 S 9. Disulfide R-S-S-R – formed when two sulfhydryls join – Family = disulfides – disulfide bonds between amino acids stabilizes protein structure Name the Family! Name the Family! aldehyde aldehyde alcohol alcohol ketone Name that Functional Group!! Name that Functional Group!! alcohol amine Carboxylic acid amine aldehyde ketone Morphine Morphine hydroxyl oxyl amine hydroxyl Epinephrine Epinephrine hydroxyl hydroxyl amine hydroxyl Maitotoxin (toxin discovered in contaminated reef fishes) Maitotoxin (toxin discovered in contaminated reef fishes) hydroxyl Maitotoxin (toxin discovered in contaminated reef fishes) hydroxyl oxyl Maitotoxin (toxin discovered in contaminated reef fishes) hydroxyl oxyl others Summary of Functional Groups • Functional Groups are specific arrangements of atoms that are attached to C chains. • Look for the nonhydrocarbon parts of the molecule • Learning goal: be able to recognize and name these 9 functional groups on organic molecules Biology 121 Lectures 2.1 & 2.2 Organic Molecules Functional Groups Chemical Reactions What is a Chemical Reaction? • Remember our concept of the hierarchal organization of life • Subatomic particles atoms molecules… • The next step in our understanding of molecules, we can add that… molecules undergo chemical changes. Chemical Reactions Rearrange Bonds • Sometimes when molecules come into contact with one another, the conditions dictate that the bonds holding those molecules together are replaced by new bonding interactions. • Simple example of a chemical reaction: Chemical Reactions Rearrange Bonds • A reaction = breaking and/or forming bonds to create different molecules • Reactants (what you start with) Products (what you end with) • Show reactions in the form of an equation A + B C + D Chemical Reactions • Later in this course, we’ll discuss specific categories of chemical reactions. • For now, it is sufficient to set the groundwork by learning how to diagram chemical reactions, and how reactions can be placed in categories based on similarities in their outcome. Diagramming Reactions • Here is a specific example of a simple chemical reaction, burning methane (CH4). CH4 is the primary component of natural gas, so this reaction is one that takes place in many furnaces. • Notice there are two reactants, they are separated with a “+” • There are two products, they are also separated with a “+” CH4 + O2 → CO2 + H2O Diagramming Reactions • There is a number “2” in front of O2 and in front of H2O, because there are two of them. For every single CH4 that burns, 2 O2molecules are needed. • Chemical reactions, written properly, have the same number of each atom on each side of the arrow. CH4+2O2→CO2+2H2O 5 Important Types of Reactions 1. Bond Rearrangement – changing the bonds within a molecule to form a new molecule – No addition or loss of atoms, just changes form one structural isomer to another 2. Functional Group Transfer – moves a functional group from one molecule to another – Phosphate groups are frequently transferred from one molecule to another 5 Important Types of Reactions 3. Dehydration/Condensation – joining two molecules by removing a water molecule – Allows creation of big macro molecules from subunits – One molecule donates an H+, one donates an OH-, and they bond to each other – CH3–CH2–OH + HO–CH2–CH3 CH3–CH2–O–CH2–CH3 + H20 – Anabolic reaction (building up molecules), takes energy • Simple sugars complex carbohydrates • Amino acids proteins 3. Dehydration/Condensation • Sugar monosaccharides are joined via dehydration/ condensation during starch synthesis • Amino acids joined this way during protein synthesis 5 Important Types of Reactions 4. Cleavage – breaking a bond to make smaller molecule – Hydrolysis – breaking a molecule into two smaller molecules using H2O – CH3–CH2–O–CH2–CH3 + H20 CH3–CH2–OH + HO–CH2–CH3 – Opposite of dehydration, catabolic reaction (breaking down molecules), released energy 5. Oxidation/Reduction – transfer of electrons between molecules – Will deal with these in detail in section 4 Enzymes assist most reactions • Reactions can happen on their own, but enzymes make them react more quickly and efficiently – Hold reactants in place in the right orientation so the reaction can occur – Almost every reaction in the body requires its own specific enzyme Learning Goals: • Describe the 4 types of reactions discussed in class and given an equation or diagram, determine which type of reaction is depicted • For dehydration and hydrolysis reactions, know if the reaction is anabolic or catabolic and whether it requires or releases energy • Understand that dehydration synthesis is the reaction that joins subunits to make complex carbohydrates, lipids, proteins, and nucleic acids