E: Kinetic-Molecular Model of an Ideal Gas
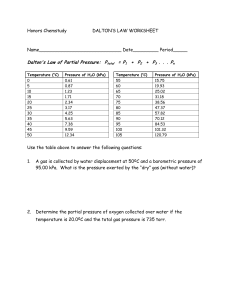
advertisement

1
PHYS1001
Physics 1 REGULAR
Module 2 Thermal Physics
PRESSURE
IDEAL GAS
EQUATION OF STATE
KINETIC THEORY MODEL
THERMAL PROCESSES
ap06/p1/thermal/ptE_gases.ppt
2
Overview of Thermal Physics Module:
1. Thermodynamic Systems:
Work, Heat, Internal Energy
0th, 1st and 2nd Law of Thermodynamics
2. Thermal Expansion
3. Heat Capacity, Latent Heat
4. Methods of Heat Transfer:
Conduction, Convection, Radiation
5. Ideal Gases, Kinetic Theory Model
6. Second Law of Thermodynamics
Entropy and Disorder
7. Heat Engines, Refrigerators
3
Kinetic-Molecular Model of an Ideal Gas
Thermal Processes
* Ideal gas, Equation of state (§18.1 p611)
* Kinetic-molecular model of an ideal (results only – not the
mathematical derivations) (§18.3 p619)
* Heating a gas: heat capacities, molar heat capacity
(§17.5 p582 §18.4 p626 §19.6 p658 §19.7 p659)
* First Law of Thermodynamics: Internal Energy, Work, Heat,
Paths between thermodynamic states
(§19.1 p 723-725, §19.2 p725-728, § 19.3 p728-729, §19.4 p729-735)
* Thermal Processes and pV diagrams: Isothermal, Isobaric
Isochoric (constant volume gas thermometer), Adiabatic Cyclic
(§19.5 p735-737, §19.8 p741-744, §17.3 p644-645
References: University Physics 12th ed Young & Freedman
4
HEAT ENGINES & GASES
5
Phases of matter
Gas - very weak
intermolecular forces,
rapid random motion
Liquid - intermolecular
high temp
low pressure
forces bind closest neighbours
Solid - strong
intermolecular forces
low temp
high pressure
6
Ideal Gas
* Molecules do not exert a force on
each other zero potential energy
* Large number of molecules
* Molecules are point-like
* Molecules are in constant random
motion
* Collisions of molecules with walls of
a container and other molecules
obey Newton's laws and are elastic
7
Quantity of a gas
number of particles N
mass of particle m
molar mass M
(kg.mol-1)
number of moles n
mass of 1 mole of a substance
( mol) 1 mole contains NA particles
Avogadro's constant NA = 6.023x1023 mol-1
1 mole is the number of atoms in a 12 g sample of carbon-12
1 mole of tennis balls would fill a volume equal to 7 Moons
The mass of a carbon-12 atom is defined to be exactly 12 u
u atomic mass units, 1 u = 1.66x10-27 kg
(1 u)(NA) = (1.66x10-27)(6.023x1023) = 10-3 kg = 1 g
mtot = N m
If
N = NA mtot = NA m = M
n = N / NA = mtot / M
M = NA m
8
1.00 kg of water vapour H2O
M(H2O) = M(H2) + M(O) = (1 + 1 + 16) g = 18 g = 1810-3 kg
n(H2O) = mtot / M(H2O) = 1 / 1810-3 = 55.6 mol
N(H2O) = n NA = (55.6)(6.0231023) = 3.351025
m(H2O) = M / NA = (1810-3) / (6.0231023) kg = 2.9910-26 kg
1 amu = 1 u = 1.6610-27 kg
m(H2O) = 18 u = (18)(1.6610-27) kg = 2.9910-26 kg
9
Pressure P
pressure !!!
Is this pressure?
What pressure is applied to the ground if a person stood on one heel?
Pressure P (Pa)
Impact of a molecule on the wall of the
container exerts a force on the wall and
the wall exerts a force on the molecule.
Many impacts occur each second and
the total average force per unit area is
called the pressure.
P=F/A
force F (N)
area A (m2)
pressure P (Pa)
Patmosphere = 1.013105 Pa
~1032 molecules strike our skin every day with an avg speed ~ 1700 km.s -1
10
11
Rough estimate of atmospheric pressure
air ~ 1 kg.m-3 g ~ 10 m.s-2 h ~ 10 km = 104 m
p = F / A = mg / A = V g / A = A h / A = g h
Patm ~ (1)(10)(104) Pa
Patm ~ 105 Pa
12
Famous
demonstration
of air pressure
(17thC) by
Otto Van
Guerickle of
Magdeburg
… and all the king's horses …
What force is required to separate the hemispheres? Is this force
significant?
?
13
Famous demonstration of air
pressure (17thC) by Otto Van
Guerickle of Magdeburg
p = 1x105 Pa
R = 0.30 m
A = 4R2
F = p A
F = (105)(4)(0.3)2 N
F = 105 N
14
Gauge and absolute pressures
Pressure gauges measure the pressure above and below
atmospheric (or barometric) pressure.
Patm = P0 = 1 atm = 101.3 kPa = 1013 hPa = 1013 millibars =
760 torr = 760 mmHg
Gauge pressure Pg
Absolute pressure P
P = Pg + Patm
Pg = 200 kPa Patm = 100 kPa
P = 300 kPa
200
100
0
300
400
15
Ideal Gases – equation of state (experimental law)
pV=nRT=NkT
must be in kelvin (K)
R, Universal gas constant
(same value for all gases)
R = 8.314 J.mol-1.K-1
Boltzmann constant
k = R / NA
k = 1.38x10-23 J.K-1
R = k NA
16
All gases contain the same number of molecules when
they occupy the same volume under the same conditions
of temperature and pressure (Avogadro 1776 - 1856)
p V = n R T n = N / NA= p V / R T
Ideal gas, constant mass (fixed quantity of gas)
p1V1
T1
p2 V2
T2
17
Boyle's Law (constant temperature)
p = constant / V
Charles Law (constant pressure)
V = constant T
Gay-Lussac’s Law (constant volume)
p = constant T
18
Isothermals pV = constant
180
160
pressure p (kPa)
140
120
p
100
n RT
V
100 K
200 K
300 K
80
400 K
60
40
20
0
0.00
0.05
0.10
0.15
0.20
0.25
3
volume V (m )
0.30
0.35
0.40
Thermodynamic system
(ideal gas)
work
internal energy
pV=nRT
pV=NkT
k = R / NA
W p dV
U = Q – W
= n CV T
p V T
U S
heat
mtot N n
mtot = n M
Q = n C T
CV or Cp
N = n NA
S
dQ
T
Q=0
p V = constant
T V-1 = constant
19
Ideal gas - equipartition of energy classical picture
- not valid at low or high temperatures
Degrees of freedom - there is kinetic energy associated
with each type of random motion
Translation f = 3
z
y
x
Vibration
only at high T
Rotation
diatomic molecule f = 2
Provided the temperature is not too high (< 3000 K), a
diatomic molecule has 5 degrees of freedom
20
21
Kinetic–Molecular model for an ideal gas (p619)
Large number of molecules N with mass m randomly
bouncing around in a closed container with Volume V.
pV n R T N k T
Experimental Law
Kinetic-Molecular Model
(Theory)
pV
z
2
KEtr
3
y
x
For the two equations to agree, we must have:
KEtr
3
3
n RT N k T
2
2
22
Total kinetic energy for random translational motion of all molecules, Ktr
KEtr
1
2
3
3
1 2
n R T N k T N mvavg
2
2
2
2
mvavg
is the average translational kinetic energy of a single molecule
Average translational KE of a molecule
1 2
1
3
mvavg m v X2 ,avg vY2 ,avg vZ2 ,avg k T
2
2
2
For an ideal gas, temperature is a direct measure of the
average kinetic energy of its molecules.
23
At a given temperature T, all ideal gas molecules have the
same average translational kinetic energy, no matter what the
mass of the molecule
energy stored in each degree of freedom = ½ k T
Theorem of equipartition of energy (James Clerk Maxwell):
The thermal energy kT is an important factor in the natural sciences. By
knowing the temperature we have a direct measure of the energy available
for initiating chemical reactions, physical and biological processes.
24
Internal energy U of an ideal gas
U
KE PE KE N f
random
U
f
N kT
2
random
U
1
kT
2
PE = 0
f
N k T
2
Degrees of freedom (T not too high)
monatomic gas,
diatomic gas,
polyatomic gas,
f=3
f = 5,
f=6
Only translation possible at
very low temp, T rotation
begins, T oscillatory
motion starts
25
Heating a gas
Q mtot c T
mtot N m
Q n M c T
Q n C T
Molar heat capacity
CMc
NM
nM
NA
26
Heating a gas at constant volume
f
Q n CV T
U N k T
2
st
1 Law Thermodynamics
U = n CV T
U Q W
f
N k T n CV T
2
n 1 N NA
CV
f
R
2
NA k R
Q
Constant volume
process V = 0 W = 0
All the heat Q goes into changing the
internal energy U hence temperature T
Larger f larger CV smaller T for a given Q
27
Heating a gas at constant pressure
f
Q n C p T U N k T n CV T
2
1st Law Thermodynamics
W
U Q W
Q
n CV T n C p T n R T
Cp CV R
Constant pressure
process W = p V
pV n R T p V n R T
It requires a greater heat input to raise the temp of a gas a given
amount at constant pressure c.f. constant volume
Q = U + W W > 0
CV
f
R
2
28
f 2
R
2
f 2
R
Cp
2 C R
2
1 V
f
CV
f
CV
R
2
CV CV R
C p CV R
CV
R
1
monatomic
diatomic
f 3 monatomic 1
f 5 diatomic 1
2
1.67
3
2
1.40
5
29
T1
Thermal processes
p1
p2
W
V1
U1
S1
V2
U2
n N mtot
pV n RT N k T
S2
Q
U Q W nCV T
C p CV R
Q n C p T
T2
Q n CV T
S 12
dQ
T
Reversible processes
Isothermal change T = 0
U = 0
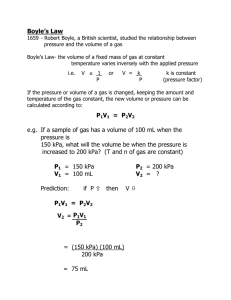
Boyle’s Law (1627 -1691)
T1= T2 p1V1 = p2 V2
pV = n R T
nRT
Q W VV2 pdV VV2
1
1 V
p1 V1 p2 V2
V W
ln 2
V1 nRT
V2 p1
V1 p2
V2
dV nRT ln
V1
p
Q W n R T ln 1
p2
W
V
2 e nRT
V1
W
p2 nRT
e
p1
30
31
Isothermal process
200
180
Isothermals pV = constant
pressure p (kPa)
160
140
120
100 K
100
400 K
80
60
800 K
1
40
2
W
20
0
0.00
0.05
0.10
0.15
0.20
0.25
3
volume V (m )
W is the area under an isothermal curve
isotherm
Isochoric (V = 0)
32
W = 0 U = Q = n CV T
Isochoric process
200
180
pressure
p (kPa)
160
140
2
1 to 2: Q > 0 T 1 < T 2
T > 0
U > 0
120
100 K
W=0
100
400 K
80
60
800 K
Isothermals pV = constant
1
40
20
0
0.00
0.05
0.10
0.15
0.20
0.25
3
volume V (m )
isochor
33
Isobaric (p = 0)
W = p V
Isobaric process
Q = n Cp T
U = Q – W = n CV T
200
180
Isothermals pV = constant
pressure
p (kPa)
160
T2 > T1 U > 0 W > 0 Q > 0 W < Q
140
120
100 K
100
400 K
80
60
V1/T1=V2/T2
2
800 K
1
40
W
20
0
0.00
0.05
0.10
0.15
3
volume V (m )
0.20
0.25
isobar
34
Adiabatic (Q = 0)
U = - W
CV = (f / 2) R Cp = CV + R = (f / 2 +1)R
= Cp / CV
= (f + 2) / f
p V = constant
diatomic gas f = 5
= 7 / 5 = 1.4
T V -1 = constant
W
CV
1
p1V1 p2 V2
p1V1 p2 V2
R
1
35
Adiabatic process
200
180
Isothermals pV = constant
pressure
p (kPa)
160
140
1 to 2: Q = 0 T1 > T2 W > 0 U < 0
120
100 K
100
400 K
800 K
80
60
1
W
W
40
2
20
0
0.00
0.05
0.10
0.15
volume V (m3)
0.20
0.25
adiabat
An adiabat steeper on a pV diagram than the nearby isotherms since > 1
36
Adiabatic processes can occur when the system is well
insulated or a very rapid process occurs so that there is not
enough time for a significant heat to be transferred eg rapid
expansion of a gas; a series of compressions and
expansions as a sound wave propagates through air.
Atmospheric processes which lead to changes in
atmospheric pressure often adiabatic: HIGH pressure cell,
falling air is compressed and warmed. LOW pressure cell,
rising air expands and cooled condensation and rain.
37
Atmospheric adiabatic processes
Q=0
U = - W
T V -1 = constant
convergence
divergence
HIGH - more uniform
conditions - inhibits cloud
formation
divergence
convergence
LOW - less uniform
conditions - encourages cloud
formation
sunshine
Burma Cyclone
5 May 2008 +50 000 killed ?
38
U W
T
T12
n CV dT p dV
dT
R V2 dV
T
CV V1 V
R Cp CV
TV
( 1)
R
R
CV
ln(T2 / T1 )
ln(V2 / V1 ) ln (V1 / V2 )
CV
Cp
R
1 1
CV CV
constant
nRT
dV
V
p1V1 p2V2
T1
T2
p2 V1
pV constant
p1 V2
T2 V1
T1 V2
( 1)
T2 p2V2 V1
T1
p1V1 V2
( 1)
Cyclic Processes:
U = 0 reversible cyclic process
39
40
Problem E.1
Oxygen enclosed in a cylinder with a movable piston
(assume the gas is ideal) is taken from an initial state A
to another state B then to state C and back to state A.
How many moles of oxygen are in the cylinder? Find
the values of Q, W and U for the paths A to B; B to C;
C to A and the complete cycle A to B to C to A and
clearly indicate the sign + or – for each process.
Does this cycle represent a heat engine?
41
100
90
C
pressure
p (kPa)
80
70
60
100 K
50
400 K
40
800 K
A
30
B
20
10
0
0.00
0.02
0.04
0.06
0.08
0.10
0.12
3
volume V (m )
0.14
0.16
0.18
0.20
Thermodynamic system
(ideal gas)
work
internal energy
pV=nRT
pV=NkT
k = R / NA
mtot = n M
N = n NA
W p dV
p V T
U S
U = Q – W
= n CV T
heat
mtot N n
Q = n C T
CV or Cp
Q=0
p V = constant
T V-1 = constant
42
43
Solution
Identify / Setup
oxygen diatomic f = 5
CV = (f / 2) R
Cp = CV + R = (f / 2 +1) R
CV = 5/2 R
Cp = 7/2 R
R = 8.315 J.mol-1.K-1
CV = 20.8 J.mol-1.K-1
Cp = 29.1 J.mol-1.K-1
pV=nRT=NkT
U n CV T
U Q W
W p dV area under pV graph
Q n CV T
Q n C p T
Execute
n
At A
4
2
p A VA 4 10 2 10
mol 0.96 mol 1 mol
RTA
8.31 102
100
90
C
pressure
p (kPa)
80
70
60
100 K
50
400 K
40
800 K
A
30
B
20
10
0
0.00
0.02
0.04
0.06
0.08
0.10
0.12
3
volume V (m )
0.14
0.16
0.18
0.20
44
45
1 A to B is isobaric
100
90
T1= TB – TA = (400 – 100) K = 300 K
C
p (kPa)
80
pressure
pA = pB = p1 = 40 kPa = 4.00104 Pa
V1 = (0.080 – 0.020) m3 = 0.060 m3
70
60
100 K
50
40
400 K
800 K
A
30
Q1
20
B
10
W1 > 0 since gas expands
0
0.00
0.02
0.04
0.06
0.08
0.10
3
U1 > 0 since the temperature increases
U1 = n CV T1 = (1)(20.8)(300) J = 6.2103 J
Q1 > 0 since U1 > 0 and U1 = Q1 – W1 > 0
Q1 = n Cp T1 = (1)(29.1)(300) J =
8.7103
0.12
volume V (m )
W1 = p1 V1 = (4.0104)(0.06) = 2.4103 J
Q1 > W1 > 0
J
Check: First law
U1 = Q1 - W1 = (8.73103 – 2.4103) J = 6.3103 J
0.14
0.16
0.18
0.20
46
100
90
C
pressure
p (kPa)
80
70
60
100 K
50
400 K
40
800 K
A
30
B
20
10
0
0.00
0.02
0.04
0.06
0.08
0.10
0.12
3
volume V (m )
0.14
0.16
0.18
0.20
47
100
2 B to C is isochoric
90
C
= 400 K
pressure
T2 = TC – TB = (800 – 400) K
p (kPa)
80
V2 = 0 m3
70
60
100 K
50
40
A
30
10
0
0.00
0.02
0.04
0.06
U2 > 0 since the temperature increases
U2 = n CV T2 = (1)(20.8)(400) J = 8.3103 J
Q2 = 8.3103 J
800 K
B
20
W2 = 0 since no change in volume
Q2 = U2 since W2 = 0
400 K
Q2
0.08
0.10
0.12
3
volume V (m )
0.14
0.16
0.18
0.20
48
100
90
C
pressure
p (kPa)
80
70
60
100 K
50
400 K
40
800 K
A
30
B
20
10
0
0.00
0.02
0.04
0.06
0.08
0.10
0.12
3
volume V (m )
0.14
0.16
0.18
0.20
49
3 C to A
T3 = TA – TC = (100 – 800) K = -700 K
pA = 40 kPa = 4.0104 Pa pC = 80 kPa = 8.0104 Pa pCA = 4.0104 Pa
VA = 0.02 m3
VC = 0.08 m3
V3 = 0.06 m3
W3 < 0 since gas is compressed
W3 = area under curve = area of rectangle + area of triangle
W3 = - { (0.06)(4.0104) + (½)(0.06)(8.0104- 4.0104)} J = - 3.6103 J
100
=-
14.6103
J
Q3 = U3 + W3
Q3 = (- 14.5103 - 3.6103) J = - 18.2103 J
C
Q3
80
p (kPa)
U3 = n CV T3 = (1)(20.8)(-700) J
90
pressure
U3 < 0 since the temperature decreases
70
60
100 K
50
40
400 K
800 K
A
30
B
20
10
0
0.00
0.02
0.04
0.06
0.08
0.10
0.12
3
volume V (m )
0.14
0.16
0.18
0.20
50
Complete cycle
U = 0 J
1
A to B
2
B to C
3
C to A
(total values)
W (kJ)
+ 2.4
0
- 3.6
- 1.2
Q (kJ)
+ 8.7
+ 8.3
- 18.2
+1.2
U (kJ)
+6.3
+8.3
- 14.6
0
Q – W (kJ)
+ 6.3
+ 8.3
- 14.6
0
Refrigerator cycle: |QH| = |QC| +|W|
TH
|QH|
cycle
|W|
|QC|
TC
Wcycle < 0 work is done on the system
the system is not a heat engine because a heat engine needs to do a net
amount of work on the surroundings each cycle.
The net work corresponds to the area unenclosed i.e. the area of the triangle:
Wcycle = - (1/2)(0.06)(4.0104) J = - 1.2103 J (value agrees with table)
100
90
C
pressure
p (kPa)
80
70
60
100 K
50
40
400 K
800 K
A
30
B
20
10
Evaluate
0
0.00
0.02
0.04
0.06
0.08
0.10
0.12
3
volume V (m )
0.14
0.16
0.18
0.20
51
52
Problem E.2 Typical 5 mark exam question
An ideal gas is enclosed in a cylinder which has a movable piston. The gas
is heated resulting in an increase in temperature of the gas and work is
done by the gas on the piston so that the pressure remains constant.
(a) Is the work done by the gas positive, negative or zero. Explain
(b) From a microscopic view, how are the gas molecules effected?
Explain.
(c) From a microscopic view, how is the internal energy of the gas
molecules effected?
(d) Is the heat less than, greater than or equal to the work? Explain.
Solution
Identify / Setup
53
p
T 2 > T1
W p dV p V2 V1 0
V2
V1
U nCV T
T2
First Law of Thermodynamics
U Q W
T1
V1
V2
V
54
(a)
Since work is done by the gas on the piston, the system expands
as the volume increases (pressure remains constant)
W p dV p V2 V1 0
V2
V1
The work done by the gas on the piston is positive.
(b)
Since the temperature increases, the average translational
kinetic energy of the gas molecules increases.
55
(c)
The change in internal energy, U of an ideal gas is given by
U n CV T
where n is the number of moles of the gas and CV is the molar heat
capacity of the gas at constant volume. Since the temperature
increases, the internal energy must increase. Therefore, the total
kinetic energy due to random, chaotic motion of the gas
molecules increases.
56
(d)
Heat Q refers to the amount of energy transferred to the gas due to
a temperature difference between the system and surroundings.
First law of thermodynamics
U Q W
U 0 Q 0 W 0 Q W
The heat Q is greater the work done by the gas W.