CULTURE METHODS
advertisement

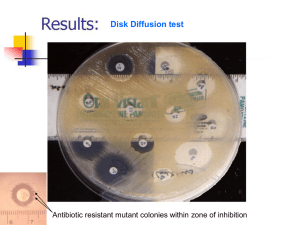
CULTURE METHODS G.HARIPRASAD M.Sc.Med Micro M.Phil LECTURER THOOTHUKUDI GOVT. MEDICAL COLLEGE THOOTHUKUDI PURPOSE OF CULTURE To isolate bacteria in pure culture. Demonstrate their properties. Obtain sufficient growth for preparation of antigens and for other tests. Type isolates by methods such as bacteriophage and bacteriocin susceptibility. Determine sensitivity to antibiotics. Estimate viable counts and Maintain stock cultures. METHODS Streak culture. Lawn culture. Stroke culture. Stab culture. Pour plate culture. Liquid culture. STREAK CULTURE Routinely used method to isolate bacteria. One loopful of culture is made as a primary inoculum and is then distributed thinly over the plate by streaking it with the loop in a series of parallel lines in different segments of the plate. Loop flamed and cooled between the different sets of streaks. On incubation growth may be confluent at the site of the original inoculation but becomes progressively thinner and well separated colonies are obtained over the final series of streaks LAWN CULTURE Also called as carpet culture. Provides a uniform, growth of the bacterium. Useful for bacteriophage typing and antibiotic sensitivity testing. Also used in the preparation of bacterial antigens and vaccines. Prepared by flooding the surface of the plate with a liquid culture or suspension of the bacterium, pipetting off the excess inoculum and incubating the plate. Alternatively the surface of the plate may be inoculated by applying a swab soaked in the bacterial culture or suspension. COTTON SWAB IS DIPPED IN CULTURE NEAR FLAME SWAB CHARGED WITH CULTURE IS SWABBED ON PLATE ANTIBIOTIC SENSITIVITY TESTING STROKE CULTURE Made in tubes containing agar slopes (slant). Employed for providing a pure growth of the bacterium for slide agglutination and other diagnostic tests. STAB CULTURE Prepared by puncturing with a long straight, charged wire in a suitable medium such as nutrient gelatin or glucose agar. Medium is allowed to set with the tube in the upright position, providing a flat surface at the top of the medium. Employed mainly for demonstration of gelatin liquefaction and oxygen requirement of the bacterium under study. Also used in the maintenance of stock culture. POUR PLATE CULTURE Tubes containing 15 ml of the agar medium are melted and left to cool in a water bath at 45ºC-50ºC. Dilutions of the inoculum are added in 1 ml volume to the molten agar, mixed well. Contents poured in sterile petri dishes and allowed to set. After incubation colonies will be seen well distributed throughout the depth of the medium. Enumerated using colony counters. Gives an estimate of the viable bacterial count in a suspension and is the recommended method for quantitative urine cultures. COLONY NUMBERS DECREASES WITH SERIAL DILUTION COLONY COUNTER LIQUID CULTURES Inoculated by touching with a charged loop or by adding the inoculum with pipettes or syringes. Method employed for blood culture & for sterility tests. Preferable for inocula containing antibiotics and other antibacterial substances. Preferred when large yields are desired. ANAEROBIC CULTIVATION METHODS To isolate anaerobic bacteria – which grow only in the absence of oxygen but grow only in the presence of carbon-di-oxide (20%) Creating Anaerobic condition called anaerobiosis established by various methods. In one way, Anaerobic condition can be created in a airtight closed container – anaerobic jar (McIntosh & Filde’s jar)- made upof either stainless steel or polycarbonate/polypropylene In another way, anaerobic condition can be created by using anaerobic media in test tubes (contain reducing substance) provide anaerobic condition. Methods establishing anaerobic condition in anaerobic jar Anaerobic gaspak method Evacuation – replacement method Anaerobic gaspak method Gas pak is commercially used and never be used again Gas-pak is a disposable packet containing pellets of sodium borohydride, cobalt chloride, citric acid and sodium bicarbonate. These chemicals generate hydrogen and carbon dioxide when water is added (about 10 ml) ( some gaspak do not need water) Hydrogen combines with oxygen in the presence of a catalyst. After the inoculated plates are placed inside an air-tight jar, the packet of “Gas-pak” with water added, is kept inside and the lid is tightly closed. METAL JAR PLASTIC JAR McIntosh jar GAS PAK CATALYST EVACUATIONREPLACEMENT METHOD This is performed by evacuating preexisting air from jar and filling of hydrogen and carbon dioxide. The anaerobic jar is provided with inlet and outlet and pressure gauze Through inlet gases are passed inside the jar – but through outlet gas is evacuated. After placing the plates and palladium catalyst the jar is tightly closed The remaining air (preexisting oxygen) is evacuated by vacuum pump First Hydrogen gas (90%) is passed, followed by passing carbon dioxide(10%) and incubated. ANAEROBIC SETUP ANAEROBIC MEDIA Robertson’s Cooked Meat (RCM) Broth: Cooked meat particles used as a reducing agent which absorbs oxygen. Anaerobes attack meat – proteolytic Eg. Cl. tetani Anaerobes attack carbohydrates in meat – saccharolyic Eg. Clostridium perfringens THIOGLYCOLLATE BROTH Thioglycollate used as a reducing agent which absorbs oxygen. Bacterial growth identified by turbidity. ANAEROBIC CULTURE METHODS 3.ABSORPTION OF CHEMICALS WITH CHEMICALS Alkaline pyrogallol – absorbs oxygen. Pyrogallic acid added to a solution of sodim hydroxide in a large test tube placed inside an air-tight jar – provides anerobiosis. QUALITY ASSURANCE Checking whether the process is working properly or not. Chemical indicators: Methylene blue indicator – turns white when reduced i.e.during anaerobiosis. Biological indicators: Pseudomonas aeruginosa ( aerobic bacteria) will grow if anaerobiosis is not maintained. CANDLE JAR METHOD:- inoculated plates are placed inside a large air-tight container and a lighted candle kept in it before the lid is sealed – provides a concentration of carbondioxide which stimulates the growth of most bacteria. References: www.slideshare.net