Types of fermentor systems…………………………………………………..
advertisement

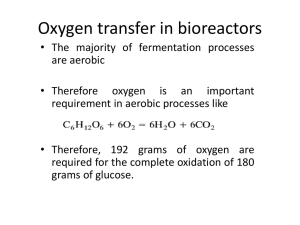
November 12 Fermentor systems 2012 SUBMITTED TO: DR ZAFFAR MEHMOOD SUBMITTED BY: TAHIRA KHAN HASSAN CHAUDHRY MADIHA HAMID KANWAL SHAHEEN AISHA NAEEM Food Biotechnology TABLE OF CONTENTS: Introduction to fermentor systems…………………………………………... 1 Fermentor design……………………………………………………………... 3 Types of fermentor systems………………………………………………….. 7 Submerged culture fermentor……………………………………………….. 9 Solid state fermentor…………………………………………………………. 15 Applications and types of Fermentors used in food industry……………… 20 Murree brewery an industrial model fermentor……………………………. 26 References……………………………………………………………………. 37 2 FERMENTER SYSTEMS Fermentation is carried out in vessels known as Fermenters. A fermenter can be a simple vessel but if it is connected to complex integrated system of automated control, then it is termed as fermenter system. HISTORY OF FERMENTATION Divided into four stages: 1. Pre 1900 2. 1900-1940 3. 1940-date 4. 1964-date 5. 1979 –date 1. First Stage: Wooden vessels were used. These wooden vessels had the capacity of approximately 1500 barrels. In the later years the trend of carrying out fermentation in copper vessels was seen. 2. Second Stage: Steel vessels were used which had the ample capacity of 200m3. Such vessels were mainly used for acetone or butanol fermentation. Air spargers were also introduced in the second stage for the aeration of yeast and mechanical stirring also came into practice for small vessels. 3. Third Stage: Mechanical aerated vessels are among the salient features of the third stage. True fermenters i.e. the ones which are operated aseptically also came into practice. 4. Fourth Stage: 3 Development of pressure cycle and pressure jet vessels occurred in fourth stage which led to the exclusion of some major problems like gas exchange and heat exchange. 5. Fifth Stage: Fermenters actually developed in the third and fourth stage. Along with fermenters animal cell reactors were also developed. FERMENTER VS BIOREACTOR Fermenter system is used for the growth and maintenance of a population of bacterial or fungal cells. While, a bioreactor is used for the growth and maintenance of a population of mammalian or insect cells. FERMENTER DESIGN 1. MATERIALS USED IN A FERMENTER: Due to the strict aseptic environment, the materials used in fermenter should be able to withstand repeated sterilizations usually done with steam. Secondly the use of appropriate material depends mainly upon the scale. For a small scale, it is a common practice to use glass or stainless steel. Glass because it has a smooth surface, it is non-toxic and corrosion proof and most importantly it is easy to examine the interior of the vessel. Pilot-scale and industrial scale vessels are normally constructed of stainless steel or at least have a stainless-steel cladding to limit corrosion. 2. CONDITIONS FOR A FERMENTER: Following conditions should be met in order to make a proper fermenter and for it to work in an efficient way. To achieve these, the fermenter should have: • Heat and oxygen transfer configuration • Impeccable sterilization procedures • Foam control • Fast and thorough cleaning system 4 • Proper monitoring and control system • Productivity and yield • Fermenter operability and reliability • Product purification • Water management • Energy requirements • Waste treatment Other few significant things to be taken in account include: • Design in features so that process control will be possible over reasonable ranges of process variables. • Operation should be reliable • Operation should be contamination free 3. STERILIZATION: A. Sterilization Of The Fermenter: Fermenters are designed in such a way so that it may be steam sterilized with pressure when necessary. Moreover the medium also needs to be sterilized either in the vessel or outside the vessel before it is added into the vessel aseptically. B. STERILIZATION OF AIR SUPPLY: Aerobic fermentation processes an ample amount of air is needed and the air should be sterilized before entering the fermenter. There are two conventional ways of sterilizing air i.e. is by heating and by filtration. Heat is discouraged because of its being too costly to implement in full-scale operation. 5 C. STERILIZATION OF THE EXHAUST GAS FROM THE FERMENTER: Exhaust gas can be sterilized by using filters of 0.2 µm placed on the exhaust pipe. Usually the exhaust gas contains moisture and solid particles leading to aerosol formation. This can be avoided by either using a cyclone operator before solids or the coalesce for liquids before the exhaust pipe opening in series. To make sure that no viable cells are leaving the exhaust pipe. The filter needs to be constantly checked. 4. SENSOR PROBES: Glass electrodes are used as sensor probes but in order to seal these probes rings are used commonly known as Double ‘O’ rings. These work as an aseptic seal and allow minimum release of micro-organisms and if in any case a leakage is inevitable then there are simple disinfection protocols to deal with it. Pre-installed back up probes are also necessary because if in a case a probe fails then there is a chance of leakage of broth if a retractable probe housing is used during the fermentation. 5. AGITATOR: Also known as impeller. It is required for the purpose of mixing, e.g. broth mixing, transfer of oxygen & heat, solid particles suspension and for maintaining a constant environment in the fermenting vessel. 6. SPARGER: Sparger is also called aeration system. It is a device for introducing air into the fermenter broth. There are three standard types of spargers namely: Porous sparger. Orifice sparger. A perforated pipe. Nozzle sparger. A partially closed pipe. 7. TEMPERATURE CONTROL: 6 Usually the heat produced by microbial activity and mechanical mixing is not enough and external must be provided or the heat is too much and it must be removed from the system. This can be done by silicone heating coils or the heating jacket through which the water is circulated. For the large scale fermenters, internal coils and cold water circulation are preferred because of the increase in surface area. OPERATION OF A FERMENTER SYSTEM Industrial fermentation processes may be carried out as batch fermentations, fed-batch operations or continuous fermentations. Batch and fed-batch operations are quite common, continuous fermentations being rare. The mode of operation is, to a large extent, dictated by the type of product being produced. BATCH FERMENTATION Batch fermentation system is a closed culture system which contains an initial, limited amount of nutrient. In batch processing, a batch of culture medium in a fermenter is inoculated with a microorganism (the ‘starter culture’) and incubation is allowed to proceed under optimal physiological conditions. In the course of the entire fermentation, nothing is added except oxygen, antifoam and acid/base to control the pH. Composition of the culture medium, biomass, and metabolite concentration change constantly as a result of the cell metabolism. The fermentation proceeds for a certain duration (the ‘fermentation time’ or ‘batch time’) and the product is harvested. zThe inoculated culture will pass through a number of phases, as shown in figure. After inoculation there is a phase during which it appears that no growth takes place; this period is referred to as the lag phase and considered as a time of adaptation. Following a period during which the growth rate of the cells gradually increases, the cells grow at maximum rate and this period is known as log phase or exponential, phase. After the substrate is exhausted, the growth ceases and this is called stationary phase. The depletion of substrate for maintenance and the presence of toxic substances cause the cell death, called death phase. Batch fermentations typically extend over 4-5 days, but some traditional food fermentations may last months. Most beer breweries use batch processes commercially. 7 FED-BATCH FERMENTATION A fed-batch is a biotechnological batch process which is based on feeding of a growth limiting nutrient substrate to a culture. The fed-batch strategy is typically used in bio-industrial processes to reach a high cell density in the bioreactor. Mostly the feed solution is highly concentrated to avoid dilution of the bioreactor. The controlled addition of the nutrient directly affects the growth rate of the culture and helps to avoid overflow metabolism (formation of side metabolites, such as acetate for Escherichia coli, lactic acid in cell cultures, ethanol in Saccharomyces cerevisiae), oxygen limitation (anaerobiosis).The volume of fermenting broth increases with each addition of the medium, and the fermenter is harvested after the batch time. CONTINUOUS FERMENTATION In continuous fermentations, sterile medium is fed continuously into a fermenter and fermented product is continuously withdrawn, so the fermentation volume remains unchanged .Typically , continuous fermentations are started as batch cultures and feeding begins after the microbial population has reached a certain concentration. In some continuous fermentations, a small part of harvested culture may be recycled, to continuously inoculate the sterile feed medium entering the fermenter. ‘Plug flow’ fermentation devices, such as long tubes that do not allow back mixing, must be inoculated continuously . Elements of fluid moving along in a plug flow device behave like tiny batch fermenter. Hence, true batch fermentation processare relatively easily transformed into continuous operations in plug flow fermenters, especially if p H control and aeration are not required. Continuous cultures are particularly susceptible to microbial contamination, but in some cases the formation conditions may be selected (e.g. low pH, High Alcohol or salt content) to favor the desired microorganisms compared to potential contaminants. 8 TYPES OF FERMENTER SYSTEMS Most commercially useful fermentations may be classified as either submerged cultures or solid state fermentations. These are the two basic types of fermenter systems, which will be discussed in detail. Solid-state and submerged fermentations may be each subdivided- into oxygen requiring aerobic processes, and anaerobic that must be conducted in the absence of oxygen. Examples of aerobic fermentations include submerged-culture citric acid production by Aspergillus Niger and solid state Koji fermentation (used in production of soya sauce). Fermented meat products such as bologna sausage (polony) dry sausage, pepperoni and salami are produced by solid state anaerobic fermenations utilizing acid-forming bacteria, particularly lactobacillus, Pediococcus and mircoococcus species. A submerged culture anaerobic fermentation occurs in yogurt making. SUBMERGED CULTURE FERMENTER SYSTEM Submerged fermentation is the cultivation of microorganisms in liquid nutrient broth. Submerged fermenter systems may use a dissolved substrate e.g. sugar solution, or a solid substrate 9 suspended in a large amount of water to form a slurry. Submerged fermentations are used for pickling vegetables, producing yoghurt, brewing beer and producing wine and soay sauce. The process involves growing carefully selected microorganisms in closed vessels containing a rich broth of nutrients (the fermentation medium) and a high concentration of oxygen. As the microorganisms break down the nutrients, they release the desired enzymes into solution. Fermentation takes place in large fermenter with volumes of up to 1,000 cubic metres. The fermentation media sterilizes nutrients based on renewable raw materials like maize, sugars and soya. Parameters like temperature, pH, oxygen consumption and carbon dioxide formation are measured and controlled to optimise the fermentation process. Firstly, in harvesting enzymes from the fermentation medium one must remove insoluble products, e.g. microbial cells. This is normally done by centrifugation. As most industrial enzymes are extracellular (secreted by cells into the external environment), they remain in the fermented broth after the biomass has been removed. The biomass can be recycled as a fertiliser, but first it must be treated with lime to inactivate the microorganisms and stabilise it during storage. Advantages: Measure of process parameters is easier than with solid-state fermentation. Bacterial and yeast cells are evenly distributed throughout the medium. There is a high water content which is ideal for bacteria. Disadvantages: High costs due to the expensive media Large reactors are needed and the behaviour of the organism cannot be predicted at times. There is also a risk of contamination 10 A typical submerged culture vessel has the features shown in the following figure: SUBMERGED CULTURE FERMENTER DESIGN: (1)Reactor Vessel (2)Jacket (3)Insulation (4)Protective Shroud (5)Inoculum Connection (6)Ports of sensors of pH, temperature and dissolved O2 (7)Agitator (8)Gas Sparger (9)Mechanical Seal (10)Reducing Gearbox (11)Motor (12)Harvest Nozzle (13)Jacket Connection (14)Sample valve with steam connection (15)Sight Glass (16)Connections of acids, alkalis and antifoam agents (17)Air inlet (18)Removable Top (19)Medium Feed nozzle (20)Air exhaust Nozzle -connect to condenser, not shown (21)Instrumentation ports for foam sensors pressure gauge and other devices (22)Centrifugal foam beaker (23)Sight glass with light-not shown and steam connection (24)Rupture disc nozzle DIFFERENT TYPES OF SUBMERGED CULTURE FERMENTERS The major types of submerged cultures fermenter systems are as follows: 1. Stirred tank fermenter 2. Air lift fermenter 3. Bubble column fermenter 11 4. Fluidized-bed fermenter 5. Trickle-bed fermenter 1. STIRRED TANK FERMENTER A stirred tank fermenter is the simplest type of fermenter system. It is composed of a reactor and a mixer such as a stirrer, a turbine wing or a propeller. This is a cylindrical vessel with working height to-diameter ratio (aspect ratio)of 3-4. A central shaft supports three to four impellers , placed about 1 impellers diameter that direct the flow axially(parallel to shaft) or radially(outwards from the shaft). Sometimes axial- and radial flow impellers are used on the same shaft. The vessel is provided by four equally spaced vertical baffles, that extend from near the walls in to vessels. Typically, the baffle width is 8-10% of the vessel diameter. This reactor is useful for substrate solutions of high viscosity and for immobilized enzymes with relatively low activity. However, a problem that arises is that an immobilized enzyme tends to decompose upon physical stirring. This system is generally suitable for the production of rather small amounts of chemicals. 2. BUBBLE COLUMN FERMENTER A bubble column fermentation system is an apparatus used for gas-liquid reactions first applied by Helmut Gerstenberg. This is a cylindrical vessel with a working aspect ratio 4-6. The 12 introduction of gas takes place at the bottom of the column and causes a turbulent stream to enable an optimum gas exchange. In this way, the compressed gas provides agitation. It is built in numerous forms of construction. The mixing is done by the gas sparging and it requires less energy than mechanical stirring. The liquid can be in parallel flow or counter-current. Bubble column reactors are used in various types of chemical reactions like wet oxidation, or as Algae bioreactor. Although simple, it is not widely used because of its poor performance relative to other systems. It is not suitable for very vicious broths or those containing large amount of solids. 3. AIR LIFT FERMENTER Air-lift bioreactors are similar to bubble column reactors, but differ by the fact that they contain a draft tube. The draft tube may be an inner tube (called "air-lift bioreactor with an internal loop”) or an external tube (called "air-lift bioreactor with an external loop”) which improves circulation and oxygen transfer and equalizes shear forces in the reactor. In the internal-loop designs the aerated riser and unaerated down corner are contained in small shell. In the external- 13 loop configuration, the riser and the down comer are separate tubes that are linked near the top and the bottom. Liquid circulates between the riser (upward flow) and the down comer (downward flow). The working aspect ratio of airlift fermenters is 6 or greater. Generally, these are very capable fermenters, except for handling vicious broths. The ability to suspend solids and transfer O2 and heat is good. The hydronamic shear is low. The external loop design is relatively little-used in industry. 4. FLUIDIZED-BED FERMENTER These are similar to bubble columns with an expanded cross section near the top. Fresh or recirculated liquid is continuously pumped into the bottom of the vessel, at a velocity that is sufficient to fluidize the solids or maintain them in a suspension.These fermenters need an external pump. The expanded section slows down the local velocity of the upward flow, such that the solids are not washed out of the bioreactors. In this type of fermenter, a fluid (gas or liquid) is passed through a granular solid material at high enough velocities to suspend the solid and cause it to behave as though it were a fluid. This process, known as fluidization, imparts many important advantages to the FBR. As a result, the fluidized bed reactor is now used in many industrial applications. 5. TRICKLE-BED FERMENTER These consist of a cylindrical vessel packed with support material (e.g wood chips, rocks, plastic structure). The support has large open spaces, for the flow of liquid and gas and the growth of 14 microoraganisms on the solid support. A liquid nutrient broth is sprayed onto the top of support material, and trickles down the bed. Air may flow up the bed, countercurrent to the liquid flow. These fermenters are used in vinegar production, as well in other process. These are suitable for liquids with low viscosity and few suspended solids. SOLID STATE FERMENTER SYSTEMS SSF involves the growth of micro organisms on moist solid substrate where there is little water in the spaces between the substrate molecules and a continuous gas phase. In the beginning it was thought that liquid state fermentation or submerged fermentation (SLF) has more advantage over SSF. But recent studies in the West have shown SSF to be the cheapest and more environmentally friendly relative to SLF in the production of value added industrial based products such as enzymes, bio fuels and the likes. However in the East it is still in the back burners of the fermentation popularity just due to poor understanding and control of SSF. Traditional uses of SSF systems include the production of fermented foods, pigments, and koji in the Far East. Within the past decade, the production of other, higher-value microbial metabolites such as antibiotics ,biopesticides , aromas, gibberellic acid and bacterial amylase to name a few, have been evaluated with highly promising results. Bread, sausages, and soy sauce are also some familiar products of SSF. Some of the advantages of SSF are listed below: 15 The use of little moisture may facilitate the production of some specific compounds which can’t be produced in SLF. The products obtained in SSF are more thermo tolerant relative to those produced in SLF. The low availability of water reduces the possibilities of contamination by bacteria and yeast. This allows working in aseptic conditions in some cases. Simply designed reactors with few spatial requirements can be used due to the concentrated substrates. DIFFERENT TYPES OF SS FERMENTERS The design of the fermenter is very important for a fermentation process. Solid substrate fermentation fermenters vary in technical sophistication from the very primitive banana leaf wrappings to highly automated machines used mainly in Japan. There are many different types of fermenters used for SSF. A few are explained below: 1. TRAY FERMENTER This fermenter is one of the simplest and widely used fermenters. Its basic part is a wooden, metal, or plastic tray, often with a perforated or wire mesh bottom to improve air circulation. A shallow layer of less than 0.15 m deep, pretreated (e.g., steamed) substrate is placed on the tray for fermentation. Temperature and humidity-controlled chambers are used for keeping the individual trays or stacks. A spacing of at least one tray height is usually allowed between stacked trays. Cheesecloth may be used to cover the trays to reduce contamination, but strict monosepticity is not attempted. Inoculation occasional mixing are and done manually, often by hand. Small- and medium- scale koji operations in Asia mostly use this technology. 16 Disadvantages: Despite some automation, tray fermenters are labor intensive require a large area Difficulties with processing hundreds of trays limit their scalability 2. STATIC BED AND TUNNEL FERMENTERS These are the modification of tray fermenter employing a single, larger and deeper, static bed of substrate with forced aeration through the bed. The substrate is located in an insulated chamber. Tunnel Fermenter: In the tunnel fermenter, the bed of solids may be quite long but is usually no deeper than 0.5 m. Tunnel fermenters may be highly automated with mechanisms for continuous feeding, mixing inoculation and harvest of substrate. 3. ROTARY-DISK FERMENTERS Rotary disk fermenters are used in large scale koji fermentations in Japan. They consist of a upper and lower chambers, each with a circular perforated disk to support the substrate. A common central shaft rotates the disks. Inoculated substrate is introduced in the upper chamber and slowly moved to the transfer screw. The upper screw transfers the partly fermented through a mixer to the lower chamber where fermentation further occurs. The mixer solids breaks up the partly fermented substrate–mycelium aggregates halfway through the fermentation process. Fermented substrate is eventually harvested using the lower transfer screw. Both chambers are aerated with humidified, temperature-controlled air. Rotating-disc contactors have been used in effluent treatment. They utilize a growing microbial film on slow rotating discs to oxidize the effluent. 17 AUTOMATIC ROTARY 4. TOWER Tower fermenter KOJI FERMENTER FERMENTOR is simple in design and easy to construct. It is similar in concept to rotary koji fermentor, consisting of a long cylindrical vessel with an inlet at the bottom, an exhaust at the top, and a jacket to control temperature. A stack of several tray chambers form the tower. It does not require agitation hence there are no shafts, impellers or blades. Tower fermentors are used for continuous fermentation of beer, yeast and SCP. In 1955 these were used in brewing industry. TOWER FERMENTOR Disadvantages: Despite of being simple and agitation free (to keep yeast cells in suspension) the tower fermentors have following drawbacks : 1. Long start up 2. Technical complexity 3. Skilled personnel Required 4. No product consistency 5. AGITATED TANK FERMENTOR Helical ribbon-stirred tank fermentors have been employed for solid-state culture of fungi such as Chaetomium cellulolyticum on wheat straw. Other similar designs have utilized multiple helical screws for agitation of large rectangular tanks. 18 6. CONTINUOUS SCREW FERMENTOR A screw fermentor is used for continuous fermentation process. Sterilized, cooled, and inoculated substrate is fed at the inlet. The screw moves the fermenting solids toward the harvest port. The fermentation time depends on the length of the screw and the rotational speed. As the device is not aerated, therefore only anaerobic or microaerophilic fermentations may be done. SCREW FERMENTOR 7. AUTOCLAVE FERMENTOR Most fermentors are sterilized by autoclaving, or hot steam under pressure. For small laboratory fermentors they are sterilized in autoclaves. In the case of large fermentors, most if not all are equipped with in situ sterilization facilities built into the fermentor system. For most autoclaving sterilizations in both small and large fermentors, the accepted autoclaving conditions is at 121 degrees Centigrade at pressure of 15 to 20 psi. The autoclaving holding time is about 15 to 20 minutes. There are certains point which should be kept in mind while autoclaving,they are as follows:i. To be efficient in autoclaving it is very important to drive off any air pockets that might be present in the autoclave. Air is a poor heat conductor. If the air is not driven out it will be difficult to bring the right temperature in all the autoclave. Let the autoclave heat and steam up and release the hot steam through an escape valve before closing the valve and starting the sterilization process. ii. Ensure that the temperature recorded in the autoclave chamber is uniform throughout the whole chamber. Make sure the temperature stated on the panel outside the autoclave is the real temperature inside the autoclave chamber. We do not want under heating and overheating to occur. Place thermo probes to measure the real temperature of the autoclave and repair if needed. iii. Do not overload the autoclaving chamber. This might lead to poor degree of sterilization being achieved. iv. Ensure that the autoclave is not leaking or suffering from leak in pressure as it will affect the sterilization process. 19 APPLICATIONS OF FERMENTORS IN INDUSTRY Industrial fermentation is the intentional use of fermentation by microorganisms such as bacteria and fungi to make products useful to humans. Fermented products have applications as food as well as in general industry. FOOD FERMENTATION Ancient fermented food processes, such as making bread, wine, cheese, curd, dosa etc., can be dated to more than 6000 yr ago. They were developed long before man had any knowledge of the existence of the microorganisms involved. Fermentation is also a powerful economic incentive for semi-industrialized countries, in their willingness to produce bio-ethanol. PHARMACEUTICALS AND THE BIOTECHNOLOGY INDUSTRY There are 5 major groups of commercially important fermentation: 1. Microbial cells or biomass as the product, e.g. single cell protein, bakers yeast, lactobacillus, E. coli, etc. 2. Microbial enzymes: catalase, amylase, protease, pectinase, glucose isomerase, cellulase, hemicellulase, lipase, lactase,streptokinase, etc. 3. Microbial metabolites : Primary metabolites – ethanol, citric acid, glutamic acid, lysine, vitamins, polysaccharides etc. Secondary metabolites-- all antibiotics fermentation 4. Recombinant products: insulin, hepatitis B vaccine, interferon, granulocyte colonystimulating factor, streptokinase 5. Biotransformations: phenylacetylcarbinol, steroid biotransformation, etc. NUTRIENT SOURCES FOR INDUSTRIAL FERMENTATION Growth media are required for industrial fermentation, since any microbe requires water, (oxygen), an energy source, a carbon source, a nitrogen source and micronutrients for growth. 20 Carbon & energy source + nitrogen source + O2 + other requirements → Biomass + Product + byproducts + CO2 + H2O + heat PRODUCTION OF INDUSTRIAL ENZYME USING DIFFERENT FERMENTORS NEUTRAL PROTEASE: Neutral protease is produced at indrustial level using agro-industrial residues as substrate e.g. wheat bran, rice husk, rice bran, spent brewing grain, coconut oil cake, palm kernel cake, sesame oil cake, jackfruit seed powder and olive oil cake etc. while developing a production medium it is very important to monitor the cost-effectiveness of the medium so these agro-industrial residues mentioned above are are very cheap and easily available. Among all substrates wheat bran is the best. Seven fungal cultures, i.e. three strains of Aspergillus oryzae and four strains of Penicillium species e.g. P. funiculosum, P. funiculosum, P. pinophilum, P. aculeatum were evaluated using a plate assay for enzymeproduction, which showed a strain of A. oryzae NRRL 1808 as the most useful culture. Protease enzyme is produced in two fermentor systems, in solidstate fermentors (SSF) and sub-merged fermentors (SmF). PRODUCTION OF NEUTRAL PROTEASE IN SSF AND IN SMF AND THEIR COMPARISON: In SSF a medium having an initial moisture content of 43.6%, when inoculated with 1 ml of spore suspension (8 × 108 spores) and incubated at 30 °C for 72 h (31.2 U enzyme per gram of fermented substrate – U/gds) is used while in SmF medium (pH 7.5) containing 2% (w/v) wheat bran, when inoculated with 3 ml of spore suspension and incubated at 30 °C and 180 rpm for 72 h gave maximum enzyme yield of 8.7 U/gds is used. SSF gives best result comparative to SmF because of 3.5-fold more enzymeproduction in SSF and it clearly demonstrating the superiority of SSF over SmF. Biesebeke et al. compared the molecular and physiological aspects of the fungus in submerged and solid-statefermentation. He observed a number of differences correlated with the different growth conditions. SmF has advantages in process control and easy recovery of extracellular enzymes, mycelia or spores. However, the products are dilute and enzymic extracts might be less 21 stable than those from SSF. SSF has been developed and described for fungal enzymeproduction and its advantages include simplicity, lower production costs, high enzyme yields and low wastewater output. SSF has the added advantage since it is a static process without mechanical energy expenditures, although problems such as temperature and pH control are encountered. GLUCOAMYLASE PRODUCTION OF GLUCOAMYLASE IN SSF: Aspergillus sp. A3 is used for the production of glucoamylase under solid state fermentation. Different substrates like wheat bran, green gram bran, black gram bran, corn flour, barley flour, jowar flour, maize bran, rice bran and wheat rawa are the best substrate and give best results among all these wheat bran showed the highest enzyme activity. The maximum enzyme activity under optimum conditions was 247 U/g of wheat bran. The optimum conditions are fructose as additive 1% w/w, urea as additive 1% w/w, incubation time of 120 h, incubation temperature at 30 °C, 2:10 (v/w) ratio of salt solution to weight of wheat bran, inoculum level 10% v/v,moisture content of solid substrate 80%, 1:50 ratio of substrate weight to flask volume and pH 5.0. SSF holds tremendous potential for the production of enzymes. In case of crude fermented product SSF is of special interest, may be used directly as enzyme source. For the SSF processes Agro-industrial residues are generally considered the best substrates. PRODUCTION OF GLUCOAMYLASE IN SMF: Currently, glucoamulase enzyme is also produce in submerged fermentation (SmF), generally employing genetically modified strains. Comparative to SSF in SmF the cost of production is high and is uneconomical. So as a result the SSF should be considered as an attractive method and it has has many advantages over submerged fermentation for the production of enzyme. 22 ALGAE BIOFUELS PHOTOBIOREACTOR Ability of microalgae to mitigate CO2 emission and produce oil with a high productivity show that it has the potential for applications of producing the third-generation of biofuels. For microalgae biofuel production there is a need of identification of preferable culture conditions for high oil productivity, development of effective and economical microalgae cultivation systems, as well as separation and harvesting of microalgal biomass and oil. Chisti in 2007 proposed that under suitable culture conditions, some microalgal species are able to accumulate up to 50–70% of oil/lipid per dry weight. And Gouveia and Oliveira in 2009 proposed tha the fatty acid profile of microalgal oil is suitable for the synthesis of biodiesel. Chisti also proposed the major reason of using microalgal oil for biodiesel production which is the tremendous oil production capacity by microalgae, as per hectare, they could produce up to 58,700 L oil, which is one or two magnitudes higher than that of any other energy crop. However, it also faces a number of technical hurdles that render the current development of the algal industry economically unfit. In addition, it is also necessary, but very difficult, to develop cost-effective technologies that would permit efficient biomass harvesting and oil extraction. Nevertheless, since microalgae production is regarded a feasible approach to mitigate global warming, it is clear that producing oil from microalgal biomass would provide significant benefits, in addition to the fuel. Photobioreactor could be effective to grow microalgae by using favorable light source and reactor configuration. Collection and concentration of microalgal biomass from cultivation systems contribute heavily to the operation cost of the overall process. METHANE FERMENTATION SYSTEM FOR FOOD RECYCLING Keeping in mind the applicability of food waste leachate (FWL) in bioreactor landfills or anaerobic digesters to produce methane as a sustainable solution to the persisting leachate management problem and this research was made in Korea. Taking into account the climatic conditions in Korea and FWL characteristics, the effect of key parameters, i.e. temperature, alkalinity and salinity on methane yield was investigated. The monthly average moisture content and the ratio of volatile solids to total solids of the FWL were found to be 84% and 91%, respectively. The biochemical methane potential experiment under standard digestion conditions 23 showed the methane yield of FWL to be 358 and 478 ml/g VS after 10 and 28 days of digestion, respectively, with an average methane content of 70%. Elemental analysis showed the chemical composition of FWL to be C13.02H23.01O5.93N1. The highest methane yield of 403 ml/g VS was obtained at 35 °C due to the adaptation of seed microorganisms to mesophilic atmosphere, while methane yields at 25, 45 and 55 °C were 370, 351 and 275 ml/g VS, respectively, at the end of 20 days. Addition of alkalinity had a favorable effect on the methane yield. Dilution of FWL with salinity of 2 g/l NaCl resulted in 561 ml CH4/g VS at the end of 30 days. Considering its high biodegradability (82.6%) and methane production potential, anaerobic digestion of FWL in bioreactor landfills or anaerobic digesters with a preferred control of alkalinity and salinity can be considered as a sustainable solution to the present emergent problem. FWL is a mechanically pretreated, easily soluble substrate, which can be handled by environmentally friendly biological practices such as landfilling and anaerobic digesters to obtain both economic and environmental co-benefits. The lab-scale BMP test showed the methane yield of FWL to be 478 ml/g VS at 35 ± 2 °C after 28 days of digestion. Methane gas in the digestion process accounted for over 70% (v/v) of the total biogas produced. Methane yield was highest (403 ml/g VS) at a mesophilic temperature of 35 °C in a period of 20 days. Alkalinity addition had positive effect on the methane yield. Dilution of FWL with salinity of 2 g/l NaCl resulted in 561 ml CH4/g VS at the end of 30 days. Taking into account its elemental composition C13.02H23.01O5.93N1 and high biodegradability (82.6%), FWL can be used as a highly desirable feedstock for methane production in bioreactor landfills or anaerobic digesters and can be treated as a sustainable solution to the present emergent problem for a clean and renewable energy resource. The Bioenergy Co. of Japan has decided to construct a power plant in Tokyo Bay to recycle food waste as part of its methane fermentation power generation project, which is part of the "Super Eco-Town Project" promulgated by the Tokyo Metropolitan Government. The plant will come on stream in fiscal 2005. Under the Food Recycling Law enacted in 2001, all business entities in the food industry are obliged to reduce or recycle food waste by more than 20 percent by 2006. Methane fermentation power generation is recommended in the law as one way to recycle food waste. 24 BUBBLE COLUMN FOR CITRIC ACID PRODUCTION Citric acid is produced from the cells of the yeast Candida guilliermondii in a bubble column, that have been immobilized by adsorption onto sawdust. At a dilution rate of 0.21 h−1 in a nitrinogen-limited medium containing glucose, a reactor productivity of 0.24 g l−1 h−1 has been achieved which is twice that observed in a batch fermenter culture using freely suspended cells. The corresponding specific production rate was 0.024 g citrate g−1 biomass h−1 while the yield was 0.1 g citrate g−1 glucose utilized. These latter values were lower than those observed using freely suspended cells, indicating that further improvements can be made to the operation of the reactor. In comparison with literature reports describing other cell immobilization techniques, adsorption onto sawdust allows similar reactor productivities while being cheap and permitting simple immobilization and reactor operation. KOJI FERMENTATION SOLID STATE Aspergillus oryzae has two glucoamylase-encoding genes, glaA and glaB, their patterns of expression are different. Expression of the glaB gene is marked in solid-state culture (koji), but low in submerged culture. To elucidate the induction mechanism of the glaB promoter in solidstate culture (koji), a fusion gene system using the glaA or glaB promoter and the Escherichia coli uidA gene encoding β-glucuronidase (GUS) is employed. The expression of glaB-GUS was induced by starch or maltooligosaccharides in a similar manner to that glaA-GUS, but other physical factors were found to be required for the maximal expression of the glaB gene in solidstate culture (koji). The time-course of glaB-GUS expression in solid-state culture (rice-koji making) suggested that its expression is induced by low water activity (Aw) of the medium and high temperature. When mycelia grown on a membrane under standard conditions were transferred to low-Aw and high-temperature conditions (membrane-transfer culture, MTC), glaB expression was markedly induced, but that of glaA was not. Additionally, glaBGUS production was induced in MTC using a membrane with smaller pore size, suggesting that a physical barrier against hyphal extension could regulate glaB expression. Under conditions found to induce glaB expression, namely, starch, low-Aw, high-temperature and physical 25 barriers, approximately 6400 U/mg-protein was obtained, equivalent to that in solid-state culture (koji). In conclusion, glucoamylase production under these induction conditions achieved in MTC reached 274 U/ml-broth, which was equivalent to the level observed in solid-state culture (koji). Northern blot analysis indicated that glaB expression was induced at the level of transcription 4 h after the transfer to the inducible conditions described above. MUREE BREWERY : AN INDUSTRIAL VISIT TO STUDY FERMENTOR SYSTEMS: AN INDUSTRIAL OVERVIEW “Murree Brewery” is an ISO 14001 Certified Company established in the year 1860 in the British era is leading beer industry in Pakistan. It has two leading manufacturing units one located in Rawalpindi and other in Hattar (KPK), Pakistan. It was established for the ever increasing demand of the beer by the personal British Raj .it is the oldest venture in Pakistan for beer market up till now. The Murree Brewery at Ghora Galli was among the first modern beer breweries established in Asia. The virtues of beer brewed from barley malt & hops as a light alcoholic beverage were not lost on the local population who rapidly became avid consumers. By the turn of the 20 century, the name "Murree" was famous for its beer in keg and bottle in the bars, beer halls and army messes of British India. Murree Beer was first awarded a medal for product excellence at the Philadelphia Exhibition in 1876, followed by numerous awards over the past 150 years, Murree brewery has potential distributors with high quality beers throughout globe. The industry has expanded its business beyond Pakistan and is available to rest of the world. MUREE BREWERY PRODUCTS Murree brewery has a wide range of products which are categorized into alcoholic and non alcoholic products but the main product of export is the beer and its different types produced there Our Premium products include 26 NON ALCOHOLIC PRODUCTS: Apple malt Peach malt Lemon malt Strawberry malt Murree Sparklets (mineral water) Cindy Malt Malt 79 ALCOHOLIC PRODUCTS: Whisky Malt whisky Beer Millennium beer(7% alcohol) Classic (alcohol 5-3%) Murree beer(4.8-4.9%) Vodka Twelve years old Single Malt Whiskies Vintage with a blend of a Scotch Grain Whisky, Silver Top Gin, Bolskaya Vodka Doctor's Brandy. 27 INDUSTRIAL VISIT OF GROUP THREE: BEER PRODUCTION BY FERMENTATION Group three visited Murree brewery on October 12 , 2012 to study the fermentor systems there and studied the beer fermentation process and each and every step involved in detail. The visit was organized by Mr.khalil who was very warm and welcoming briefed us each and every step of the beer production in detail and made us possible to study the fermentor system and its aspects in detail. In order to understand the process we must look in to account the concept of brewing that is beer fermentation from barley which can be defined as Fermentation in brewing is the conversion of carbohydrates to alcohols and carbon dioxide or organic acids using yeasts, bacteria, or a combination thereof, under anaerobic conditions. A more restricted definition of fermentation is the chemical conversion of sugars into ethanol. The equation is as follows: C6H12O6 → 2 C2H5OH + 2 CO2 28 BEER FERMENTATION THE PROCESS The basic ingredients of beer are water, barley(starch source) able to be fermented (converted into alcohol),brewer's yeast to produce the fermentation and flavoring agents such as hops. Barley seeds of particular quality are imported from Australia and are graded for the production of a particular product they are categorized as grade A, grade B, grade C. The first place in the brewery which we visited was the brew house to observe the malting which is broken into three steps germination of barley and its mashing and germination. The process is categorized into four steps: 2.Steeping 3.Gemination 1.Malting 4.Mashing FIG 1 and 2 depict the germinating barley in the brew house of Murree brewery in the first step of beer production (malting) 29 MALTING: The process of making barley grains ready for the process of brewing is known as malting. STEEPING: The seed is soaked in water in a vat and aerated for 1 to 1.5 days for sprouting to activate the enzyme at a low temperature from 10-12`C. barley seeds are graded as A, B and C depending upon the type of moisture(40-45% moisture ) grade A(beer) and grade B seeds (malt) production. GERMINATION: The germination of seeds is carried out in brew house for five days where barley seeds are aerated and maintained at particular temperatures during day 1 and 2(18-19`C). After germination is done the seeds converted to malt by the process of chitting .In chitting the seends are germinated with sprouts on them . KILINING: The germinated seeds are taken to the kiln and the sprouted seeds are roasted at 180`C in a kiln. The kiln at Murree brewery was really of old and antique style .kilning basically modifies the germinating seeds by enhancing flavors and modification of barley seeds for malt and other products production. MASHING: Mashing is the process of combining a mix of milled grain (typically malted barley with supplementary grains such as corn, sorghum, rye or wheat), known as the "grain bill", and water, known as "liquor" which is used to crush malt in a vessel called a "mash tun" with the help of heating. Mashing allows the enzymes in the malt to break down the starch in the grain into sugars, typically maltose to create a malty liquid called wort. Mashing of barley produces wort .hard water is maintained at 180`C and the crushed malt is transferred to a lautering unit. 30 LAUTERING PROCESS: Lautering is the separation of the wort (the liquid containing the sugar extracted during mashing) from the grains which is done in a lauter turn at 75`C temperature .The seed is separated from the extract and un dissolved extract is removed. WORT BOILING: Boiling of malt extracts is called as wort. It basically ensures its sterility, and thus prevents a lot of infections. During the boil hops are added, which contribute bitterness, flavor, and aroma compounds to the beer. The wort is boiled at 100`C and unsuspended phenols and extract is pumped to the boiler. The boil lasts between 15 and 120 minutes, depending on its intensity, the hop addition schedule, and volume of water the brewer expects to evaporate. The wort is separated on the basis of specific gravity and it is clarified aerated and cooled then transferred to the fermentor for beer production. Fig1(a) Lautering turn Fig1(C)Temperature And Pressure Control Unit fig1(b) wort separator. Fig1(D) Wort Boiler 31 BEER FERMENTATION BATCH FERMENTATION: Murree brewery uses the open batch fermentation process to ferment beer from yeast. The yeast strain of saccharomyces cerevisiae imported from Germany which is grown in the form of stock solutions to be added to the fermentor. The yeast stock solution is made in 100ml beakers and their serial dilutions are made from 1to 10 liters the inoculums is then transferred to the Fermentors in a batch. Yeast require one day for activation which is maintained at the temperature of (14-16`C). The process is known as yeast publication .fermentation produces beer of different categories depending upon the wort density. FERMENTOR DESIGN: Murree brewery had 24 multiple open batch bubble column fermentor systems in their fermentation unit for beer production. Fermentors installed at Murree brewery was a cylindroconical vessel or CCVs, with a conical bottom and a cylindrical top lined inside with thermocouple to maintain the temperature and temperature sensor at the bottom and a pressure regulating unit as well. The cone's aperture of the batch fermentor was typically around 60°at this angle it allows the yeast to flow towards the cone's apex, but is not so steep as to take up too much vertical space. This type of confirmation handles both the fermenting and conditioning in the same tank. Fermentor was made up of stainless steel lined with a layer of concrete with a size 32 of one hectoliter (100liters). At the end of fermentation, the yeast and other solids which have fallen to the cone's apex can be simply flushed out of a port at the apex. Fermentation takes place of the wort in the presence of yeast to beer. After fermentation beer is obtained depending upon the density of the wort. The beer with 12% alcohol content is used to make classic beer and millennium beer, with (6-7%) and light or Murree beer has low content of alcohol. BEER CONDITONING AND STORAGE After initial or primary fermentation, the beer is now conditioned, matured or aged in one of several ways, which can take from 2 to 4 weeks, several months, or several years, depending on the type of beer. The beer is usually transferred into a second container, to a trub which is free from dead yeast, secondary metabolites and other undesirable flavors that are a result of primary fermentation . PACKAGING: Packaging is putting the beer into the containers in which it will leave the brewery. Typically, this means putting the beer into bottles, aluminium cans and kegs, but it may include putting the beer into bulk tanks for high-volume customers. RESEARCH PLANNING AND DEVLOPMENT LAB We visited research planning and quality control lab of Murree brewery as well our team met their quality control manager Mr Muhammad Sohail he briefed us about the fermenting problems., batch , new product ranges , future products and quality control problems. The monitoring of the products at each step to avoid any contamination. The yeast culture is checked at the end of every batch of beer and malt before packaging. Random sampling and OCU are done to check the quality of their products. The whole batch is pasterurized at 20`c then 40 `C 70`C for thirty seconds and labeled in their specialized packaging unit. FUTURE PRODUCTS: Big peach Cola whisky leechi malt pineapple malt 33 34 Fig 1. Batch fermentor systems 2. An open batch fermentor 3. Group 3 with Fermentors 4. Yeast publication unit 35 PACKAGING AND STORAGE: 36 REFERENCES: 1. BROWN, W. E. and PETERSON, W. H. (1950) Factors affecting production of penicillin in semi-pilot plant equipment Ind. Eng. Chern. 42, 1769-1774. 2. COWAN C.T. and THOMAS, C. R (1988) Materials of construction in the biological process industries. Process biochem.23(1,)5-11. 3.Cubberly, W.H., UNTERWElSER, P. M. BENJAMIN, D., KIRK-PATRICK, C.W.,KNOLL, V. and NlEMAN, K. (1980) Stainless steels in corrosion service. In Metals Handbook (9th edition), Vol.3, pp. 5693. American Society for Metals, Ohio. 4. DUURKOOP, A. (1992) Stainless steel reflects the quality of Applikon’sbioreactors. Bioteknowledge (Applikon, Holland), 12(1), 4-6. 5. APPLIKON (1991) Silicone heating jacket for temperature control. Bioteknowledge (Applikon, (1), 9-1 6. CHAIN, E. B., PALADINO, S., UGOLINO, F. CALLOW, D. S. and VAN DER SLUIS, J. (1954) A laboratory fermenter for vortex and sparger aeration. Rep. Inst. Sup. Sanita. Roma (Eng!. Ed.), 17,61-120. 7. FINN, R.F. (1954) Agitation-aeration in the laboratory and in industry. Bacteriol. Rev. 18, 254-274. 8. GORDON, J. J., GRENFELL, E., KNOWLES, E., LEGG, B. J. MCALLISTER, R. C. A. and WHITE, T. (1947) Methods for penicillin production in submerged culture on a pilot scale. 1. Gen. Microbiol. 1, 171-186. 9. HESSELINK, P. G. M., KASTELlEM, J. and LoGTENBERG, M. T. (1990) Biosafety aspects of biotechnological processes: testing and evaluating equipment and components. In Fennentation Technology: Industrial Applications, pp. 378-382 (Editor Yu, P. L.). Elsevier, London. 10. HAMBLETON, P., GRIFFITHS, B., CAMERON, D. R. and MELLING, J. (1991) A high containment polymodal pilot-plant- design concepts. 1. Chern. Tech. Biotechnol. 50, 167-180. 11. HERBERT, D., PHIPPS, P. J. and TEMPEST, D. W. (1965) Thechemostat: design and instrumentation. Lab. Practice 14, 1150-116l. 12. JACKSON, T. (1958) Development of aerobic fermentation processes: penicillin. In Biochemical Engineering, pp. 185-221 (Editor Steel, R). Heywood, London. 13. LEAVER, G. and HAMBLETON, P. (1992) Designing DlOreactors to minimize or prevent inadvertant release into the work place and natural environment. Phannac. Technol. (April), 18-26. 37 14. SOLOMONS, G. L. (1980) Fermenter design and fungal growth. In Fungal Biotechnology, pp. 55-80 (Editors Smith, J. E., Berry, D. R. and Kristiansen, B). Academic Press, London. 15. STEEL, R. and MILLER, T. L. (1970) Fermenter design. Adv. Appl. Microbiol. 12, 153-188. 16. SPIVEY, M. J. (1978) The acetone-butanol-ethanol fermentation. Process Biochem. 13(11), 2-4, 25. 17. WALKER, P. D., NARENDRANATHAN, T. J., BROWN, D.C. WOODHOUSE, F. and VRANCH, S. P. (1987) COlnta:mmlent of micro-organisms during fermentation and a01.vm;treall1 processing. In Separation for Biotechnology, pp. 469-479 (Editors Verrall, M. S. and Hudson, M. 1.). Ellis Horwood, Chichester. 18. WERNER, R. G. (1992) Containment in the development and manufacture of recombinant DNAderived products. In Safety in Industrial Microbiology and Biotechnology, pp. 190-213 (Editors Collins, C. H. and Beale, A JJ. Butter-worth-Heinemann, London. 19. PARKER, A (1958) Sterilization of equipment, air and media. In Biochemical Engineering-Unit Processes in Fomentation, pp. 94-121 (Editor Steel, R.) Heywood, London. 20. MULLER, Rand KIESLlCH, K (1966) Technology of the microbiological preparation of organic substances. Aflgl1'anre Chem. (Int. Ed.) 5, 653-662. 21. RICHARDS, J. W. (1968) Design and operation of aseptic fermenters. In Introduction to Industrial Sterilization., pp. 107-122. Academic Press, London. 22. SAJO BASHIR ET AL (2011), Microbiological Features of Solid State Fermentation and its Applications - An overview, vol no:6, pp: 21-26 . 23. http://wiki.answers.com/Q/What_are_tower_fermenters#ixzz28V4zYOZg 24.NASEER B. JAHARGIRDAR,fermentor designs,dept of microbiology. 25. DR BOULEVARD,2008 <<http://fermentationtechnology.blogspot.com/2008/01/effects-ofautoclaving-on-fermentors.html>> 26.DAVID A. MITCHELL, SOLID STATE FERMENTATION,ENZYME PRODUCTION, FOOD ENRICHMENT ,pp:2447-2450. 27. alabev.com The Ingredients of Beer. Retrieved 29 September 2008 38 28.Nachel, Marty (31 March 2008). Homebrewing For Dummies. John Wiley & Sons. p. 51. Retrieved 18 April 2012. 29. john Hall, Wolfgang David Lindell (7 October 2011). The Oxford Companion to Beer. Oxford University Press. p. 563. Retrieved 18 April 2012. 30. Dasgupta, Amitava (16 April 2011). The Science of Drinking: How Alcohol Affects Your Body and Mind. Rowman & Littlefield. p. 6. Retrieved 18 April 2012. 31. John Hall, Wolfgang David Lindell (7 October 2011). The Oxford Companion to Beer. Oxford University Press. p. 564. Retrieved 18 April 2012. 32. Ensminger 1994, p. 188 33. http://www.scribd.com/doc/16171926/Kinetics-Study-Batch-Fermentation-of-Bakers-Yeast 34. Hui & Smith 2004 35. Keith Thomas (7 October 2011). The Oxford Companion to Beer. Oxford University Press. Retrieved 16 July 2012. 36. Yahoo! Finance. Archived from the original on 2 October 2007. Retrieved 5 November 2007. 37. http://www.scribd.com/doc/48369336/Submerged-fermentation 38. http://www.crcnetbase.com/doi/abs/10.1201/9781420027976.ch1.03 39. http://www.rpi.edu/dept/chem-eng/Biotech-Environ/IMMOB/stirredt.htm 40. http://userpages.umbc.edu/~xkang/ENCH772/air-lift.html 41. http://www.massey.ac.nz/~ychisti/FerProd.html 42.Brewers Association. 2012. Retrieved 21 June 2012. 43.www.murreebrewery.com/ 39 ntrol Unit Fig1(D) Wort Boiler 40