PAIN AND DRUGS USED FOR RELIEF OF PAIN
advertisement

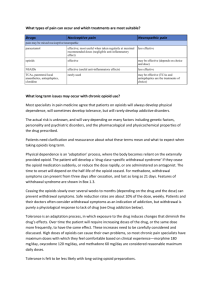
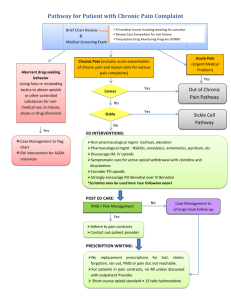
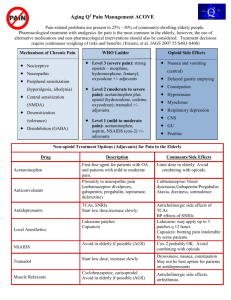
PAIN AND DRUGS USED FOR RELIEF OF PAIN /. PAIN IS THE MOST COMMON REASON PATIENTS SEEK MEDICAL CARE. Pain has sensory and emotional components and is often classified as acute or chronic. Acute pain is frequently associated with anxiety and hyperactivity of the sympathetic nervous system (e.g. tachycardia, increased respiratory rate and BP, diaphoresis, dilated pupils). Chronic pain does not involve sympathetic hyperactivity but may be associated with vegetative signs (e.g. fatigue, loss of libido, loss of appetite) and depressed mood. People vary considerably in their tolerance for pain. PATHOPHYSIOLOGY Acute pain, which usually occurs in response to tissue injury, results from activation of peripheral pain receptors and their specific A delta and C sensory nerve fibers (nociceptors). Chronic pain related to ongoing tissue injury is presumably caused by persistent activation of these fibers. Chronic pain may also result from ongoing damage to or dysfunction of the peripheral or central nervous system (which causes neuropathic pain) (see Many pain syndromes are multifactorial. For example, chronic low back pain and most cancer pain syndromes have a prominent nociceptive component but may also involve neuropathic pain (due to nerve damage). NOCICEPTIVE PAIN may be somatic or visceral. Somatic pain receptors are located in skin, subcutaneous tissues, fascia, other connective tissues, periosteum, endosteum, and joint capsules. Stimulation of these receptors usually produces sharp or dull localized pain, but burning is not uncommon if the skin or subcutaneous tissues are involved. VISCERAL PAIN receptors are located in most viscera and the surrounding connective tissue. Visceral pain due to obstruction of a hollow organ is poorly localized, deep, and cramping and may be referred to remote cutaneous sites. Visceral pain due to injury of organ capsules or other deep connective tissues may be more localized and sharp. Although pain of predominantly psychologic origin is far less common than nociceptive or neuropathic pain, psychologic factors commonly contribute to chronic pain and may contribute to pain-related disability. Pain thought to be caused predominantly by psychologic factors is sometimes called psychogenic pain; however, psychophysiologic pain is a more accurate term because the pain results from interaction of physiologic and psychologic phenomena. This type of pain can be categorized in terms of defined somatoform disorders (eg, chronic pain disorders, somatization disorders, hypochondriasis Pain fibers enter the spinal cord at the dorsal root ganglia and synapse in the dorsal horn. From there, fibers cross to the other side and travel up the lateral columns to the thalamus and then to the cerebral cortex. Repetitive stimulation (eg, from a prolonged painful condition) can sensitize neurons in the dorsal horn of the spinal cord so that a lesser peripheral stimulus causes pain (windup phenomenon). Peripheral nerves and nerves at other levels of the CNS may also be sensitized, producing long-term synaptic changes in cortical receptive fields (remodeling) that maintain exaggerated pain perception. Substances released when tissue is injured, including those involved in the inflammatory cascade, can sensitize peripheral nociceptors. These substances include vasoactive peptides (eg, calcitonin gene-related protein, substance P, neurokinin A) other mediators (eg, prostaglandin E2, serotonin, bradykinin, epinephrine ) The pain signal is modulated at multiple points in both segmental and descending pathways by many neurochemical mediators, including endorphins (e.g. enkephalin) and monoamines (e.g. serotonin, norepinephrine). These mediators interact in poorly understood ways to exaggerate or reduce the perception of and response to pain. They mediate the potential benefit of CNS-active drugs (e.g. opioids, antidepressants, anticonvulsants, membrane stabilizers) that interact with specific receptors and neurochemicals in the treatment of chronic pain. Psychologic factors are important modulators. They not only affect verbal expression of pain (i.e. whether patients appear stoic or sensitive) but also generate neural output that modulates neurotransmission along pain pathways. Psychologic reaction to protracted pain interacts with other CNS factors to induce long-term changes in pain perception. TYPES OF PAIN Physicians and neuroscientists generally classify pain in the following ways: Acute pain is caused by an injury to the body. It warns of potential damage that requires action by the brain, and it can develop slowly or quickly. It can last for a few minutes to six months and goes away when the injury heals. Chronic pain persists long after the trauma has healed (and in some cases, it occurs in the absence of any trauma). Chronic pain does not warn the body to respond, and it usually lasts longer than six months. Cancer (or malignant) pain is associated with malignant tumors. Tumors invade healthy tissues and exert pressure on nerves or blood vessels, producing pain. Cancer pain can also be associated with invasive procedures or treatments. Some physicians classify cancer pain with chronic pain. PAIN INFORMATION FROM THE FACE Your face has its own mini spinal-cord system called the trigeminal nerve. Somatosensory neurons (and pain receptors all over the face and head) travel into the central nervous system through the trigeminal nerve. They synapse in the trigeminal nucleus (group of neurons) in the mid-medulla and also on neurons in the lower medulla. Then these neurons send signals through the tregeminal-thalamic tract within the midbrain to the thalamus. Neurons in the thalamus relay signals to the somatosensory cortex and limbic system. NSAIDs: Risk of ulcers and GI bleeding due to NSAIDs for people > 65 is 3 to 4 times higher than that for middle-aged people. Risk depends on drug dose and duration of therapy. Elderly patients at high risk of GI adverse effects may benefit from concomitant use of cytoprotective drugs (usually, a proton pump inhibitor; occasionally, the prostaglandin misoprostolSome The newly recognized risk of cardiovascular toxicity, which presumably occurs with nonselective COX-1 and COX-2 inhibitors and with selective COX-2 inhibitors (coxibs), is particularly relevant to the elderly, who are more likely to have cardiovascular risk factors (eg, a history of MI or cerebrovascular or peripheral vascular disease). These drugs should be prescribed cautiously for such patients. Both nonselective and selective NSAIDs can impair renal function and cause Na and water retention; they should be used cautiously in the elderly, particularly in those who have a renal or hepatic disorder, heart failure, or hypovolemia. Rarely, NSAIDs cause cognitive impairment and personality changes in the elderly. causes more confusion in the elderly than other NSAIDs and should be avoided. Given the overall greater risk of serious toxicity in the elderly, low doses of NSAIDs should be used if possible, and using short-term therapy or interrupted therapy to confirm effectiveness should be considered OPIOIDS: In the elderly, opioids have a longer half-life and possibly a greater analgesic effect than in younger patients. Opioid agonist-antagonists often have psychotomimetic effects (eg, delirium) in the elderly and should usually be avoided. Opioids can contribute to constipation and urinary retention in patients of any age, but the effects tend to be more problematic in the elderly. Clinicians should evaluate the cause, severity, and nature of the pain and its effect on activities and psychologic well-being. Evaluation of the cause of acute pain (eg, back pain, chest pain—see elsewhere in The Manual) differs from that of chronic pain The history should include the following information about the pain: Quality (e.g. burning, cramping, aching, deep, superficial, boring, shooting) Severity Location Radiation pattern Duration Timing (including pattern and degree of fluctuation and frequency of remissions) Exacerbating and relieving factors The patient's level of function should be assessed, focusing on activities of daily living (e.g. dressing, bathing), employment, avocations, and personal relationships (including sexual). THE PATIENT'S PERCEPTION OF PAIN can represent more than the disorder's intrinsic physiologic processes. What pain means to the patient should be determined, with emphasis on psychologic issues, depression, and anxiety. Reporting pain is more socially acceptable than reporting anxiety or depression, and appropriate therapy often depends on sorting out these divergent perceptions. Pain and suffering should also be distinguished, especially in cancer patients; suffering may be due as much to loss of function and fear of impending death as to pain. Whether secondary gain (external, incidental benefits of a disorder—e.g. time off, disability payments) contributes to pain or pain-related disability should be considered. The patient should be asked whether litigation is ongoing or financial compensation for injury will be sought. A personal or family history of chronic pain can often illuminate the current problem. Whether family members perpetuate chronic pain (e.g. by constantly asking about the patient's health) should be considered. Patients and sometimes family members and caregivers should be asked about the use, efficacy, and adverse effects of prescription and OTC drugs and other treatments and about alcohol and recreational or illicit drug use. adapted from the American Geriatrics Society (AGS) Panel on Chronic Pain in Older Persons: The management of chronic pain in older persons. Journal of the American Geriatrics Society 46:635–651, 1998; used with permission; from Gloth FM III, Scheve AA, Stober CV, et al: The functional pain scale (FPS): Reliability, validity, and responsiveness in a senior population. Journal of the American Medical Directors Association 2 (3):110–114, 2001; and from Gloth FM III: Assessment. In Handbook of Pain Relief in Older Adults: An Evidence-Based Approach, edited by FM Gloth III. Totowa (NJ), Humana Press, 2003, p. 17; used with permission; copyright © FM Gloth, III, 2000. SOME PAIN SCALES FOR QUANTIFYING PAIN AS IT IS OCCURRING. For the Functional Pain Scale, examiners should clearly explain to the patient that functional limitations are relevant to the evaluation only if they are due to the pain being evaluated; treatment aims to relieve pain as much as possible, at least to a tolerable level (0–2). A -Demented and aphasic patients: Assessing pain in patients with disorders affecting cognition, speech, or language (e.g. dementia, aphasia) can be difficult. Pain is suggested by facial grimacing, frowning, or repetitive eye blinking. Sometimes caregivers can describe behaviors that suggest pain (e.g. sudden social withdrawal, irritability, grimacing). Pain should be considered in patients who have difficulty communicating and who inexplicably change their behavior. Many patients who have difficulty communicating can communicate meaningfully when an appropriate pain scale is used. Function Pain Scale has been validated and can be used in nursing home patients who have Mini-Mental State Examination scores of ≥ 17. Patients receiving neuromuscular blockade: No validated instruments are available to assess pain when neuromuscular blockade is used to facilitate mechanical ventilation. If the patient is given a sedative, the dose can be adjusted until there is no evidence of consciousness. In such cases, specific analgesics are not needed. If, however, the patient is sedated but continues to have evidence of consciousness (e.g. blinking, some eye movement response to command), pain treatment should be considered based on the degree of pain usually caused by the condition (e.g. burns, trauma). If a potentially painful procedure (eg, turning a bedbound patient) is required, pretreatment with the selected analgesic or anesthetic should be given. Last full review/revision February 2007 by Russell K. Portenoy, MD Content last modified November 2005 TREATMENT OF PAIN Nonopioid and opioid analgesics are the main drugs used to treat pain. Antidepressants, anticonvulsants, and other CNS-active drugs may also be used for chronic or neuropathic pain and are first-line therapy for some conditions. Neuraxial infusion, nerve stimulation, injection therapies, and neural blockade can help selected patients. Cognitive-behavioral interventions (e.g. incremental gains in function; changes in relationships in the home; systematic use of relaxation techniques, hypnosis, or biofeedback; graduated exercise) may reduce pain and pain-related disability and help patients cope. Pain relief may be provided parenterally. Non-opioids do not cause physical dependence or tolerance. Class Indoles Propionic acids Drug Diclofenac Etodolac Indomethacin Sulindac Tolmetin 200–400 mg q 6–8 h 25–50 mg q 6–8 h 150–200 mg q 12 h 200–400 mg q 6–8 h Fenoprofen 200–600 mg q 6 h Flurbiprofen Ibuprofen 25–50 mg q 6–8 h Naproxen 250–500 mg q 12 h Aspirin Choline Mg trisalicylate Oxicam 50–200 mg q 12 h 400 mg q 4 h to 800 mg q 6 h Ketoprofen Naproxen Na Oxaprozin Salicylates Usual Dosage Range* 50–100 mg, followed by 50 mg q 8 h 75–550 mg q 12 h 600–1200 mg q 24 h 650–1000 mg q 4–6 h 870 mg q 12 h Diflunisal 250–500 mg q 8–12 h Salsalate Piroxicam 750–2000 mg q 12 h 20–40 mg q 24 h Fenamates Meclofenamate Mefenamic acid 50–100 mg q 6–8 h 250 mg q 6 h Pyrazole Phenylbutazone 100 mg q 6–8 h up to 7 days Pyrrolo-pyrrolo derivative Ketorolac 15–30 mg IV or IM q 6 h or 20, followed by 10 mg q 4–6 h for maximum 5 days Selective COX-2 inhibitor Celecoxib 100–200 mg q 12 h NSAIDS INCLUDE NONSELECTIVE COX (COX-1 AND COX-2) INHIBITORS AND SELECTIVE COX2 INHIBITORS (COXIBS); all are 1. effective analgesics 2. least expensive but Has prolonged antiplatelet effects. Coxibs have lowest risk of ulcer formation and GI upset. However, when a coxib is used with low-dose aspirin, it may have no GI benefit over other NSAIDs. Recent studies suggest that inhibition of COX-2, which occurs with both nonselective COX inhibitors and coxibs, is associated with a prothrombotic effect that can increase risk of MI, stroke, and claudication. This effect appears to be drug-related, as well as dose- and duration-related. Although there is some evidence that the risk is very low with some of the non-selective COX inhibitors (e.g. ibuprofen naproxen) It is prudent to consider the potential for prothrombotic effects as a risk of all NSAID therapy. NSAID is likely to be used only short-term, significant adverse effects are unlikely, regardless of the drug used. Some clinicians use a coxib first whenever therapy is likely to be long-term (e.g, months) because the risk of GI adverse effects is lower. Others limit coxib use to patients A. predisposed to GI adverse effects (e.g, the elderly, patients taking corticosteroids B. with a history of peptic ulcer disease or GI upset due to other NSAIDs) C. who are not doing well with nonselective NSAIDs or who have a history of intolerance to them. Although data are still limited, the prothrombotic risk suggests that all NSAIDs should be used cautiously in patients with clinically significant atherosclerosis or multiple cardiovascular risk factors. All NSAIDs should be used cautiously in patients with renal insufficiency. Coxibs are not renal-sparing. If initial recommended doses provide inadequate analgesia, a higher dose is given, up to the conventional safe maximum dose. If analgesia remains inadequate, the drug should be stopped. If pain is not severe, another NSAID may be tried because response varies from drug to drug. It is prudent during long-term NSAID therapy to monitor for occult blood in stool and changes in CBC, electrolytes, and hepatic and renal function. OPIOID ANALGESICS “Opioid” is a generic term for natural or synthetic substances that bind to specific opioid receptors in the CNS, producing an agonist action. Opioids are also called narcotics. Some opioids used for analgesia have both agonist and antagonist actions. Potential for abuse among those with a known history of abuse or addiction may be less with agonistantagonists than with pure agonists, but agonist-antagonist drugs have a ceiling effect for analgesia and induce a withdrawal syndrome in patients already physically dependent on opioids. In general, acute pain is best treated with short-acting pure agonist drugs, and chronic pain is best treated with longer-acting pure agonist drugs During long-term treatment, the effective opioid dose can remain constant for prolonged periods. Some patients need intermittent dose escalation, typically in the setting of physical changes that suggest an increase in the pain (e.g. progressive neoplasm). Fear of tolerance should not inhibit appropriate early, aggressive use of an opioid. If a previously adequate dose becomes inadequate, that dose must usually be increased by 30 to 100% to control pain. Non-opioid analgesics (eg, , NSAIDs) are often given concomitantly. Products containing both drugs are convenient, but the non-opioid may limit upward titration of the opioid dose. OPIOID ANALGESICS ARE USEFUL IN MANAGING SEVERE ACUTE OR CHRONIC PAIN. They are often underused, resulting in needless pain and suffering because clinicians often 1. underestimate the required dosage, 2. overestimate the duration of action and risk of adverse effects 3. have unreasonable concerns about addiction Physical dependence (development of withdrawal symptoms when a drug is stopped) should be assumed to exist in all patients treated with opioids for more than a few days. However, addiction (loss of control, compulsive use, craving and use despite harm) is very rare in patients with no history of substance abuse. Before opioid therapy is initiated, clinicians should ask about risk factors for abuse and addiction. These risk factors include prior alcohol or drug abuse, a family history of alcohol or drug abuse, and a prior major psychiatric disorder. If risk factors are present, treatment may still be appropriate; however, the clinician should use more controls to prevent abuse (e.g. small prescriptions, frequent visits, no refills for “lost” prescriptions) or should refer the patient to a pain specialist or an addiction medicine specialist experienced in pain management. ROUTE OF ADMINISTRATION: Almost any route can be used. The oral or transdermal route is preferred for long-term use; both are effective and provide stable blood levels. Modified-release oral and transdermal forms allow less frequent dosing, which is particularly important for providing overnight relief. Formulations of fentanyl are now available for delivery through the oral mucosa. Lozenges are used for sedation in children and as treatment of breakthrough pain. Effervescent tablets are available for breakthrough pain. Breakthrough pain has been targeted by these formulations because they have a relatively more rapid onset than the oral route; other rapid-onset, transmucosal formulations of fentanyl and other drugs are in development. The IV route provides the most rapid onset and thus the easiest titration, but duration of analgesia is short. Large, rapid fluctuations in blood levels (bolus effect) can lead to toxicity at peak levels early in the dosing interval or later to breakthrough pain at trough levels. Continuous IV infusion, sometimes with patient-controlled supplemental doses, eliminates this effect but requires an expensive pump; this approach is used most often for postoperative pain. The IM route provides analgesia longer than IV but a. is painful b. absorption can be erratic c. not recommend. Long-term continuous sc infusion can be used, particularly for cancer pain. Intraspinal opioids (e.g. 5 to 10 mg epidurally or 0.5 to 1 mg intrathecally for acute pain) can provide relief, which is prolonged when a hydrophilic drug like is used; they are typically used postoperatively. Implanted infusion devices can provide long-term neuraxial infusion. These devices can also be used with other drugs (e.g. local anesthetics, clonidine . Dosing and titration: Initial dose is modified according to the patient's response. it is increased incrementally until analgesia is satisfactory or adverse effects limit treatment. Sedation and respiratory rate are monitored when opioids are given parenterally to relatively opioid-naive patients. The elderly are more sensitive to opioids and are predisposed to adverse effects.Opioid-naive elderly patients typically require lower doses than younger patients. Neonates, especially when premature, are also sensitive to opioids, because they lack adequate metabolic pathways to eliminate them. For moderate, transient pain, an opioid may be given prn. For severe or ongoing pain, doses should be given regularly, without waiting for severe pain; supplemental doses are given as needed when treating cancer pain and are typically considered case by case when treating chronic non-cancer pain. A common error is prescribing short-acting drugs at long intervals, allowing breakthrough pain. For patient-controlled analgesia, a bolus dose -typically 1 mg q 6 min is provided when patients push a button; a baseline infusion (e.g. morphine 0.5 to 1 mg/h) may or may not be given. The physician controls the amount and interval of the bolus. Patients with prior opioid exposure or with chronic pain require a higher bolus and baseline infusion dose; the infusion dose is further adjusted based on response. DURING LONG-TERM TREATMENT, the effective opioid dose can remain constant for prolonged periods. Some patients need intermittent dose escalation, typically in the setting of physical changes that suggest an increase in the pain (e.g. progressive neoplasm). Fear of tolerance should not inhibit appropriate early, aggressive use of an opioid. If a previously adequate dose becomes inadequate, that dose must usually be increased by 30 to 100% to control pain. Non-opioid analgesics (e.g, NSAIDs) are often given concomitantly. Products containing both drugs are convenient, but the non-opioid may limit upward titration of the opioid dose. RESPIRATORY DEPRESSION is rare with conventional doses and with long-term use. If it occurs acutely, ventilatory assistance may be needed until the opioid's effect can be reversed by an opioid antagonist. For urinary retention, double voiding or using Credé's method during voiding may help; some patients benefit from adding an α-adrenergic blocker such as tamsulosin 0.4 mg po once/day (starting dose). Opioids can cause neuroendocrine effects, typically reversible hypogonadism. Symptoms may include fatigue, loss of libido, infertility due to low levels of sex hormones, and, in women, amenorrhea. ADVERSE EFFECTS: In opioid-naive patients, adverse effects common at the start of therapy include sedation, mental clouding, constipation, nausea, vomiting, and itching. Respiratory depression is serious but is rare when opioids are given at appropriate doses. Because steady-state plasma levels are not approached until 4 to 5 half-lives have passed, drugs with a long half-life (particularly and ) have a risk of delayed toxicity as plasma levels rise. Modified-release opioids typically require several days to approach steady-state levels. In the elderly, opioids tend to have more adverse effects (commonly, constipation and sedation or mental clouding). Opioids may cause urinary retention in men with benign prostatic hyperplasia. Although tolerance to opioid-induced sedation, mental clouding, and nausea usually develops within days, tolerance to opioid-induced constipation and urinary retention usually occurs much more slowly. Any adverse effect may be persistent in some patients and this is much more likely with constipation. Opioids should be used cautiously in patients with certain disorders: Hepatic disorders because drug metabolism is delayed, particularly with modified-release preparations COPD because respiratory depression is a risk Some neurologic disorders, such as dementia and encephalopathy, because delirium is a risk Severe renal insufficiency because metabolites may accumulate and cause problems; accumulation least likely with fentanyl and methadone Constipation is common among patients who take opioids for more than a few days. For prevention in predisposed patients (e.g. the elderly), dietary fiber and fluids should be increased, and a stimulant laxative (e.g. senna) Persisting constipation can be managed with Mg 90 mL po q 2 to 3 days, lactulose 15 mL po bid, or propylethylene glycol powder (dose is adjusted as needed). Some patients require regular enemas. While sedated after taking an opioid, patients should not drive and should take precautions to prevent falls and other accidents. If sedation impairs quality of life, certain stimulant drugs may be given intermittently (e.g. before a family gathering or other event that requires alertness) or, to some patients, regularly. Drugs that can be effective are (initially, 5 to 10 mg po bid), (initially, 2.5 to 10 mg po bid), or modafinil (initially, 100 to 200 mg po once/day). These drugs are typically given in the morning and as needed later. The maximum dose of methylphenidate seldom exceeds 60 mg/day. For some patients, caffeine-containing beverages provide enough stimulation. Stimulants may also potentiate analgesia. Nausea can be treated with 25 to 50 mg po q 6 h, 10 to 20 mg po q 6 h, or an antiemetic phenothiazine (eg, prochlorperazine 10 mg po or 25 mg rectally q 6 h). Respiratory depression is rare with conventional doses and with long-term use. If it occurs acutely, ventilatory assistance may be needed until the opioid's effect can be reversed by an opioid antagonist. For urinary retention, double voiding or using Credé's method during voiding may help; some patients benefit from adding an α-adrenergic blocker such as tamsulosin 0.4 mg po once/day (starting dose). Opioids can cause neuroendocrine effects, typically reversible hypogonadism. Symptoms may include fatigue, loss of libido, infertility due to low levels of sex hormones, and, in women, amenorrhea. OPIOID ANTAGONISTS: Opioid antagonists are opioid-like substances that bind to opioid receptors but produce little or no agonist activity. They are used mainly to reverse symptoms of opioid overdose, particularly respiratory depression. Naloxone acts in < 1 min when given IV and slightly less rapidly when given IM. It can also be given sublingually or endotracheally. Duration of action is about 60 to 120 min. However, opioid-induced respiratory depression usually lasts longer than the duration of antagonism; thus, repeated doses of naloxone and close monitoring are necessary. The dose for acute opioid overdosage is 0.4 mg IV q 2 to 3 min prn. For patients receiving long-term opioid therapy, naloxone should be used only to reverse respiratory depression and must be given more cautiously to avoid precipitating withdrawal or recurrent pain. A reasonable regimen is 1 mL of a dilute solution (0.4 mg in 10 mL saline) IV q 1 to 2 min, titrated to adequate respirations (not alertness). Nalmefene is similar to naloxone, but its duration of action is about 4 to 8 h. Nalmefene is occasionally used to ensure prolonged opioid reversal. Naltrexone, an orally bioavailable opioid antagonist, is given as adjunctive therapy in opioid and alcohol addiction. It is long-acting and generally well-tolerated. ADJUVANT ANALGESIC DRUGS Many drugs are used as adjuvant analgesics, including anticonvulsants (e.g. pregabalin, gabapentin) antidepressants (eg, tricyclics, du;loxetine venlafaxine Bupropion and many others ) These drugs have many uses, most notably to relieve pain with a neuropathic component. Gabapentin is the most widely used drug for such purposes. The dose often needs to be high, up to 1200 mg tid or sometimes higher. Pregabalin is similar to gabapentin but has more stable pharmacokinetics; some patients who do not respond well to gabapentin do respond to pregabalin and visa versa. Duloxetine is a new mixed mechanism (serotonin and norepinephrine ) reuptake inhibitor, which has good evidence of analgesic efficacy in diabetic neuropathic pain and fibromyalgia. DRUGS FOR NEUROPATHIC PAIN Class/Drug Dose* Comments Carbamazepine 200–400 mg bid Monitor WBCs when starting treatment GabapentinS 300 mg bid–1200 mg tid Preferred drug in this class; starting dose usually 300 mg once/day Phenytoin 300 mg once/day Limited data; 2nd-line drug Pregabalin 75–300 mg bid Mechanism similar to gabapentinS but more stable pharmacokinetics Valproate 250–500 mg bid Limited data, but strong support for treatment of headache AmitriptylineSome 10–25 mg at bedtime May increase dose to 75–150 mg over 1–2 wk, particularly if significant depression is present; may not need high doses; not recommended for the elderly or patients with a heart disorder because it has strong anticholinergic effects Desipramine 10–25 mg at bedtime Better tolerated than amitriptyline May increase dose to 150 mg or sometimes higher Duloxetine 30 mg bid Better tolerated than tricyclic antidepressants Clonidine 0.1 mg once/day Also can be used transdermally or intrathecally Tizanidine 2–20 mg bid Less likely to cause hypotension than clonidine ANTICONVULSANTS† ANTIDEPRESSANTS CENTRAL Α2-ADRENERGIC AGONISTS DRUGS FOR NEUROPATHIC PAIN -CONTD CORTICOSTEROIDS Dexamethasone 0.5–4 mg qid Used only for pain with an inflammatory component Prednisone 5–60 mg once/day Used only for pain with an inflammatory component NMDA-RECEPTOR ANTAGONISTS Memantine 10–30 mg once/day Limited evidence of efficacy Dextromethorphan 30–120 mg qid Usually considered 2nd-line ORAL NA CHANNEL BLOCKERS Mexiletine 150 mg once/day to 300 mg q 8 h Used only for neuropathic pain For patients with a significant heart disorder, cardiac evaluation considered before the drug is started Lidocaine5% tid tid, under occlusive dressing if possible Daily Some evidence of efficacy in neuropathic pain and arthritis Usually considered for a trial if lidocaine patch is ineffective; expensive Available as patch OTHER Baclofen 20–60 mg bid Pamidronate 60–90 mg/mo May act via GABAB receptor Helpful in trigeminal neuralgia; used in other types of neuropathic pain Evidence of efficacy in complex regional pain syndrome TOPICAL Capsaicin 0.025–0.075% EMLA® *Route is oral unless otherwise indicated. †Newer anticonvulsants have fewer adverse effects. EMLA = eutectic mixture of local anesthetics; GABA = γ-aminobutyric acid; NMDA = N-methyl-d-aspartate. NEURAL BLOCKADE Interrupting nerve transmission in peripheral or central pain pathways via drugs or physical methods provides short-term and sometimes long-term relief. Neuroablation (pathway destruction is used rarely; it is typically reserved for patients with an advanced disorders and a short life expectancy. Local anesthetic drugs (eg, lidocaine) can be given IV, intrathecally, intrapleurally, transdermally, sc, or epidurally. Epidural analgesia using local anesthetics or opioids is particularly useful for some types of postoperative pain. Long-term epidural drug administration is occasionally used for patients with localized pain and a short life expectancy. Generally, for long-term neuraxial infusion, an intrathecal route via an implanted pump is preferred. NEUROABLATION Involves interrupting a nociceptive pathway surgically or using radiofrequency energy to produce a lesion. The procedure is used mainly for cancer pain. Somatic pain is more responsive than visceral pain. Neuroablation of the ascending spinothalamic tract (cordotomy) is usually used; it provides relief for several years, although numbness and dysesthesias develop. Neuroablation of the dorsal roots (rhizotomy) is used when a specific dermatome can be identified. NEUROMODULATION Stimulation of neural tissues may decrease pain, presumably by activating endogenous pain modulatory pathways. The most common method is transcutaneous electrical nerve stimulation (TENS), which applies a small current to the skin. Also, electrodes may be implanted along peripheral nerves or along the dorsal columns in the epidural space. Stimulation of brain structures (deep brain stimulation and motor cortex stimulation) has also been used, but evidence of benefit is slight. TRADITIONAL MEDICINES USED IN THE TREATMENT OF PAIN HOW DO THEY ACT? e.g. Cloves for tooth ache ACUPUNCTURE