Lecture II
advertisement
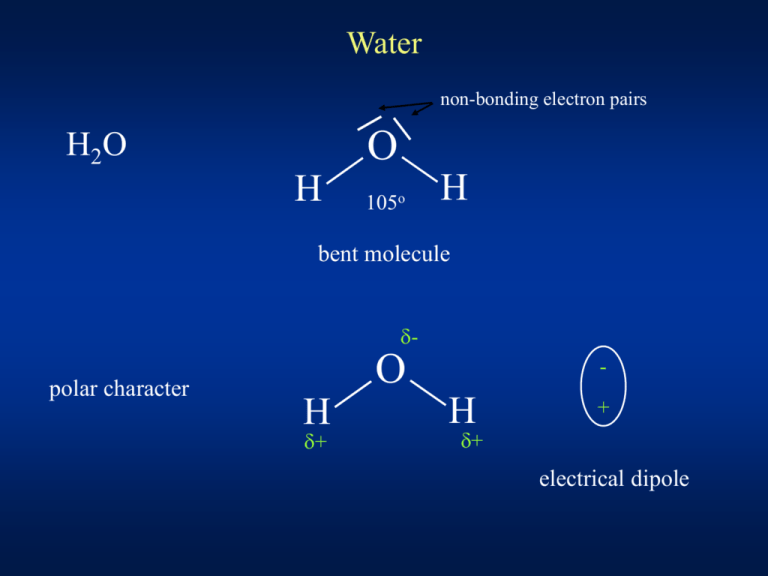
Water non-bonding electron pairs O H2O H 105o H bent molecule d- polar character O H d+ - H + d+ electrical dipole Hydrogen bonding - attractions between water molecules + + + - + - + 0 oC 3.6 / H2O HIGH melting point boiling point specific heat heat of vaporization surface tension H bonds - much weaker than covalent bonds ! bond energy of H-bonds in liquid water: about 19 kJ/mol ( covalent H – O bond in water: 460 kJ/mol ) water is most dense at 3.98 °C !!! (when freezing, ice will form first on the surface) WATER - very good solvent ! Attraction between water dipoles and ions "hydrated ions" Dispersing "amphipathic" molecules "micelles" "Amphipathic" molecules – contain both highly hydrophobic and highly polar groups palmitic acid non-polar chain = hydrophobic polar group = hydrophilic (water-fearing) (water-loving) Phospholipids phosphatidylcholine (lecithin) polar groups Biological membranes - separate the cells separate the spaces (compartments) of the cell cell membrane mitochondria nucleus lysosome endoplasmic reticulum Phospholipid BILAYER hydrophilic hydrophobic hydrophilic Water in human body ~ 60 % of the body weight intracellular ~ 40 % extracellular ~ 20 % PLASMA 5% interstitial fluid 15 % - a very complex solution of inorganic & organic components Phosphates Proteins K+ Na+ Mg2+ Na+ Cl- Na+ K+ Ca2+ HCO3- Mg2+ Phosphates K+ Na+/K+ pump ( = Na+/K+ -ATPase ) Water balance Intake: ~ 1.5 - 2.0 l / day metabolic water (produced in human body by oxidation of food) 0.3 – 0.5 l /day Resorption: stomach, small intestine, LARGE INTESTINE Secretion: Excretion: Saliva Stomach Bile Pancreas Small intestine 1 500 ml / day 2 500 500 700 3 000 Urine 60 % Skin 20 % (perspiration) Lungs 15 % Faeces 5 % 8 200 ml /day Human blood plasma mmol/l Na+ 132 – 145 K+ 3.8 - 5.2 Ca* 2.1 – 2.6 Mg2+ 0.8 – 1.1 Cl- 97 – 108 HCO3- 22 – 26 Phosphates HPO42- + H2PO4- 0.6 – 1.6 pH = 7.36 – 7.44 * Ca "total Ca" Ca2+ "ionized" is about ½ of "total" ~ 1.2 mmol/l Blood plasma ( extracellular fluid ) Cell fluid (cytosol) ( intracellular fluid ) phospholipids cholesterol PROTEINS - enzymes SELECTIVE permeability (membrane = barrier) - receptors - transport systems The flow of molecules and ions between the cell and environment is precisely regulated by SPECIFIC TRANSPORT SYSTEMS They regulate cell volume, ionic composition, pH They concentrate metabolic fuels and building blocks from the environment The extrude toxic substances They generate IONIC GRADIENTS essential for the excitability Transport across membrane 1) Passive transport "channels" 2) Active transport "pumps" - does not involve energy - diffusion from high to low concentration - uses energy (ATP) - can transport against the concentration gradient - one direction, high specificity ATP 3) Secondary active transport - no direct need of energy - gradient created by active transport is used ! Passive transport concentration and electrochemical GRADIENT 1 2 3 1) Simple diffusion toward equilibrium small molecule, NO charge, solubility in lipids ! O2 CO2 2) Ion channels - pore-forming proteins - can be "GATED" "gate" is closed 3) Facilitated diffusion ("carrier proteins") "gate" is open - selective ! - large molecules, insoluble in lipids Ion transport antibiotics Gramicidin - peptide: 15 amino acids HELIX - hydrophilic groups inside "wet channel" - lipophilic groups outside increase of the permeability of bacterial cell wall inorganic ions can travel through equilibration of concentrations = NO GRADIENT "wide wet pore" Valinomycin hydrophillic groups INSIDE = "WET cave" hydrophobic (lipophillic) groups OUTSIDE ( = soluble in lipids of membrane) K+ highly selective carrier for K+ K+ ( Na+ with water coating is too big) equilibration of K+ concentration = NO GRADIENT K+ Valinomycin hydrophillic groups hydrophobic (lipophillic) groups Active transport = "PUMPS" Transport against GRADIENT ENERGY ! ATP ADP + Pi Na+/K+ ATPase (sodium potassium pump) ATP INHIBITION: cardiotonic steroids 2 K+ 3 Na+ Na+/K+ ATPase in cell membrane of EVERY human cell ! electrogenic = transfers 3 Na+ out and ONLY 2 K+ into the cell inner side of the membrane inhibitors of this pump: - outer side of the membrane + cardiotonic steroids = cardiac glykosides (oubain, digoxin) treatment of heart failure, cardiac arrhytmia Digitalis purpurea (foxglove) H+/K+ ATPase stomach gastric acid CO2 * carboanhydrase Zn2+ HCl CO2 + H2O * H2CO 3 HCO3- H+ Cl- K+ ATP HCO3- + H+ Clblood pH = 7.4 parietal cell 106 increase of H+ concentration ! lumen of the stomach pH = 1 - 2 Secondary active transport "COTRANSPORT" Na+ dependent transport of glucose, aminoacids, Ca2+ "energy" = Na+ gradient generated by Na+/K+ ATPase Na+ glucose SYMPORT ANTIPORT Na+ Ca2+ Na+ - glucose symport glucose glucose Na+ K+ Na+ ATP proximal tubulus of each nephron in the kidneys resorption of glucose intestines resorption of glucose from GI tract Na+/Ca2+ antiport = sodium–calcium EXCHANGER Ca2+ Ca2+ Na+ K+ Ca2+ ATPase Na+/Ca2+ exchanger Na+ ATP very low concentration of Ca2+ in cytosol Endocytosis Large (polar) molecules – cannot pass through the hydrophobic membrane phagocytosis - cell ingests large object such as bacteria pinocytosis - uptake of solutes and molecules such as proteins receptor-mediated endocytosis – specific ! LDL receptor chylomicron remnant receptor receptors that mediates endocytosis of blood plasma lipoproteins cell absorbs material by engulfing it with its own membrane Exocytosis = the opposite of endocytosis Exocytosis is needed for: - secretion of large molecules from cells: glands peptide hormones B cells antibodies - neuronal chemical synapses: realease of the neurotransmitter receptor vesicles with neurotransmitter synaptic cleft Water transport across membranes Water moves by "simple diffusion" through membranes ? Additional mechanism for water transport: AQUAPORINS "water channels" Water transport - due to osmotic differences (osmotic gradient) OSMOSIS high osmolality H2O low osmolality The bioelements (summary) 1) Principal bioelements: O, C, N, H, P, S ( biomolecules: proteins, nucleic acids, lipids, saccharides ) 2) Water and ions ( H2O ) Na+, K+, Mg2+, Ca2+, Cl-, ( HCO3- , phosphates) 3) Mineral constituents of bones and teeth 4) Microelements (trace elements) Ca PO43- Fe, Cu, Co, Zn, I, F, Se, ... -------------------------------------------------------- 5) Contamination (intoxication): Hg, Al, ... Elements of group I IA Alkali metals IB H Hydrogenium Cu Cuprum Li Lithium Ag Argentum Na Natrium Au Aurum K Kalium Rb Cs Fr Alkali metals - very reactive - react with air O2 and H2O - must be stored under oil Hydrogen H biogenic element - it is present in almost all organic compounds ! H2O H+ = proton ( H3O+ ) pH = - log [H+] The pH scale 0 acidic solutions 7 neutral alkaline (basic) sol. 14 water is weakly ionized: H+ + OH- H2O KW = [H+] x [OH-] = 10-14 mol2 / l2 ionic product of water pH + pOH = 14 pure water: [H+] = [OH-] = 10-7 mol/l pH = pOH = 7 ----------------------------------------------------------------------Strong acids - fully ionised: monobasic acid: HCl dibasic acid: H2SO4 Weak acids – do not disociate completely: Strong bases: NaOH, KOH, Ca(OH)2 Weak base: NH4OH CH3COOH H+ + Cl2 H+ + SO42H+ + CH3COO- human blood plasma: pH = 7.40 +- 0.04 gastric juice: pH = 1 – 2 pancreatic juice: pH = ~ 8 ----------------------------------------------------H+ very low concentration in blood plasma !!! pH = 7.40 H+ = 0.000 040 mmol/l (40 nmol/l) (Na+ 142 mmol/l K+ 4.5 mmol/l) extreme influence of H+ on biological systems !!! ionisation of functional groups in PROTEINS Ionisation of amino acids Lithium compounds: LiCl Li2CO3 Li crimson (red) colour of flame Therapy of manic-depressive psychosis (bipolar affective disorder) = alternating periods of mania (euphoria) and depression The manic phase - increased activity, decresased need for sleep - persistent elevated mood - impaired normal functioning ! The depressive phase - lack of energy - pessimistic - self-destructive behavior (risk of suicide !) Li+ changes of ion transport in CNS - still in use "mood stabilizing agent" Lithium mineral water – therapy of GOUT (type of arthritis) (Li-urate more soluble than uric acid) Uric acid - in humans - the end product of purine catabolism OH N N HO OH N NH - poorly soluble in water - lithium urate – more soluble ! Sodium Na+ Na (Natrium) the main EXTRAcellular cation (132 – 145 mmol/l) Na+ strongly binds water ionic diameter: Na+ K+ !!! in hydrated form: Na+ larger in diameter than K+ Na+ ( together with Cl- ) large fraction of osmotic pressure (osmolality) of body fluids Water and Na balance are closely interdependent ! NaCl daily intake: 5 – 15 g Elimination: urine (95 %) sweat (perspiration) stool food (common salt) Kidney Glomerular filtration BLOOD Glomerular filtrate (180 l H2O / day) [ 1.5 kg NaCl ] Tubular resorption dependent on hormones (aldosteron, ADH) URINE 2 l H2O 5 – 15g NaCl Hormones regulating tubular resorption Aldosteron - steroid hormone (mineralocorticoid) - produced in the adrenal gland (adrenal cortex) - acting in the distal tubule of the kidney nephron: reabsorption of Na+ into blood secretion of K+ into urine Vasopressin = antidiuretic hormone (ADH) - peptide hormone - synthesized in the hypothalamus, released into blood in the pituitary gland (posterior part) - ADH increases the permeability of the collecting duct to water allows water reabsorption small volume of concentrated urine deficiency of ADH: DIABETES INSIPIDUS - polyuria - excretion of large amounts of diluted urine ( 10 – 20 l /day !) Potassium K+ K the main INTRAcellular cation (Kalium) (cytosol > 100 mmol/l) - human blood plasma: only 3.8 - 5.2 mmol/l - daily intake: ~ 4g of KCl excretion: URINE - proper concentrations of K+ and Na+ functions of membranes „membrane potential“ most cells – membrane potential relatively stable neurons, muscle cells – use changes of membrane potential for function ! (nervous system – communication between neurons) action potential Na+/K+ ATPase EXTRAcellular fluid cell membrane INTRAcellular fluid 3Na+ 2K+ Ion channels Na+ ATP ! 2K+ K+ rising phase = depolarisation ACTION POTENTIAL falling phase = repolarisation resting potential In cells K+ is bound to GLYCOGEN ! Diabetic coma ( glucose in blood ) insulin Glycogen synthesis Binding of K+ in cells Plasma K+ depletion HEART failure !!! Copper Cu (Cuprum) microelement (in human body 100 - 150 mg) dietary intake: ~ 2 mg / day Cu2+ - cofactor of some enzymes: cytochrom c oxidase (metalloenzymes) superoxide dismutase - cofactor of HEME biosynthesis CERULOPLASMIN - transport of Cu2+ in blood plasma ( 8 Cu2+ / mol. ) - a2 globulin synthesized in the liver - enzymatic activity: Fe2+ Fe3+ WILSON‘s DISEASE (hepatolenticular degeneration) - accumulation of copper in tissues - low ceruloplasmin levels - hereditary disease - neurological symptoms, liver disease CuSO4 . 5 H2O copper (II) sulphate pentahydrate = „blue vitriol“ - in Fehling‘s solution (detection of glucose in urine) copper salts - poisonous ! ------------------------------------------- Hemocyanins - Cu2+ containing proteins - O2 transport - MOLLUSCA (snail, clam, mussel, ...) - ARTHROPODA (crabs) Silver precious metal Ag ( + H 2S (Argentum) Ag2S black ! ) AgBr - photosensitive photography ------------------------------------------------------------------ Ag - useful in dental alloys for fittings and fillings ( Ag + Hg amalgam ) AgNO3 - caustic effect treatment of warts Bartholin’s gland abscess in women (removal: silver nitrate stick insertion) - diluted solution: antiseptic properties it was dropped into newborn‘s eyes to prevent gonococcal conjuntivitis ! (Gonorrhoea is a venereal disease caused by the bacteria Neisseria gonorrhoeae) Gold precious metal Au (Aurum) chemically and biologically resistent, inert in nature – almost exclusively in the native state pure gold – soft ! in jewellery: alloys (+ Cu, + Ag) harder The gold content of gold alloys in carats or in thousandths pure gold: 24 carats = 1000/1000 The standard for high quality jewellery: 18 carats = 750 / 1000 What is the gold content (g) of a 100 g piece marked 18 carats? 18 24 x 100g = 75 g Elements of group II II A Alkaline earth metals II B Be Beryllium Zn Zincum Mg Magnesium Cd Cadmium Ca Calcium Hg Hydrargyrum Ba Barium Sr Strontium Ra Magnesium Mg PLANTS: Mg2+ - central atom of green pigment CHLOROPHYLL (photosynthesis) in human body: ~ 20 g Mg > ½ in bones ( Ca-Mg phosphates ) intracellular cation Mg2+ activates number of enzymes !!! ENZYMES using ATP "kinases" hexokinase glucose glucose–6–phosphate ATP ADP (Enzymes of ATP-dependent reactions require Mg2+ as cofactor) other effects of Mg2+: anti-convulsive effect (MgSO4 - prevention of eclamptic convulsions) influence on neuromuscular excitability can help to prevent kidney and gall stones "duodenal reflex" - MgSO4 delivered into the region of the sphincter of Oddi relaxation of the sphincter + contraction of the gallbladder expulsion of bile to intestine ( the bile release from the gallbladder is stimulated by Mg2+ ) Magnesium mineral water (Karlovy Vary) purgative effect Calcium CaCO3 "burning" limestone, chalk CaO + H2O Ca CaO + CO2 quicklime "slaking" Ca(OH)2 slaked lime Hardening of mortar: Ca(OH)2 + CO2 CaCO3 + H2O Calcium Ca in human body ~ 1 kg (99% in bones, teeth) dietary intake: 800 - 1200 mg / day human blood plasma: "total Ca" (extracellular !) ionized Ca2+ 2.5 mmol/l 1.2 mmol/l resorption: ileum – specific protein carrier ~ 200 mg / day excretion: urine liver bile feces Mineral constituents of bones and teeth Hydroxyapatite Ca5 (PO4)3 OH Ffluoroapatite enamel dentin cementum pulp Enamel: - hardest substance of the body water 1-3 % organic comp. 1% mineral > 95 % (bones ~ 60 %) Hormones regulating Ca metabolism Parathormone - peptide hormone ( 84 amino acids ) - secreted by parathyreoid glands PTH - activation of bone mineral degradation Ca2+ release from bones - stimulation of Ca2+ readsorption in kidney - stimulation of calcitriol formation (kidneys) - stimulation of Ca-resorption protein formation (ileum) Ca2+ in blood Calcitonin - peptide hormone ( 32 amino acids ) - produced by parafollicular cell of the thyreoid gland - inhibition of bone mineral degradation (decrease of „osteoclasts“ activity) - stimulation of Ca2+ excretion in kidney Ca2+ in blood Salmon calcitonin is used for the treatment of OSTEOPOROSIS Calcitriol = 1,25-dihydroxycholecalciferol - active form of D–vitamin - stimulation of Ca-resorption protein formation absorption of calcium from the gastrointestinal tract PTH Ca – FOOD (protein-bound) ILEUM Ca-resorption protein calcitriol calcitonin PLASMA BONES PTH PTH URINE Ca 2.5 mmol/l Ca2+ 1.2 mmol/l calcitonin calcitriol milk excrements Ca2+ increase PTH release inhibition Very low concentration of Ca2+ in cytoplasma 10-6 mol/l MUSCLE - Ca2+ is stored in sarcoplasmic reticulum (SR) CALSEQUESTRIN = calcium-binding protein of the SR 40 Ca2+ binding sites Ca2+ in cytoplasma can cause the specific action of the cell: MUSCLES contraction Ca2+ = important SECOND MESSENGER endoplasmic reticulum "SIGNAL" release of Ca2+ EFFECT Clotting of BLOOD Intrinsic pathway Extrinsic pathway (Contact activation pathway) (Tissue factor pathway) Xa Prothrombin Thrombin Fibrinogen Fibrin Ca2+ is required for the proper function of the coagulation CASCADE Removing of Ca2+ = NO clotting ! Anticoagulants - bind Ca2+ ions ( "in vitro" = outside the body) Oxalate COO COO - Citric acid + Ca2+ COO Ca COO EDTA = ethylenediamine tetraacetic acid Gypsum, plaster of Paris CaSO4 . 2 H2O heating CaSO4 . 1/2 H2O + H 2O hardening CaSO4 . 2 H2O When the dry plaster powder is mixed with water, it re-forms into gypsum bandage impregnated with plaster = support for broken bones Strontium Sr - similar to Ca2+ incorporation in BONES (naturally present in bones in trace amounts) - new treatment for osteoporosis: „Strontium ranelate“ (improves bone density and strenght) excess: Strontium rickets radioisotope 90 Sr bone marrow irradiation LEUKEMIA half life 28 years (important isotope regarding health impacts after the Chernobyl disaster) radioisotope 89 Sr half life 50 days - treatment of bone cancer Barium Ba toxic heavy metal water-soluble compounds [ Ba(NO3)2 BaCl2 ] - strongly neurotoxic - painful cramps, tremor BaSO4 (barium sulphate) - almost insoluble in water ! - radiocontrast agent for X-ray imaging ( "barium meal" ) - imaging of the gastrointestinal tract large intestine Zinc microelement Zn (Zincum) dietary intake: 12 -15 mg / day cofactor of many ENZYMES: carboxypeptidase - protein digestion carbonic anhydrase H2O + CO2 H2CO3 H+ + HCO3- alcohol dehydrogenase - oxidation of ethanol to acetaldehyde NAD+ CH3CH2OH NADH+H+ CH3CHO Insulin binds ZINC ! "Zn – insulin hexamers" Metallothionein - protein synthesized in kidneys rich of -SH groups - can bind metals ( Zn2+ Cd2+ .... ) - zinc transport, heavy metal detoxification (Hg2+) ? Zinc is an essential nutrient for proper sperm production ! -----------------------------------------------compounds used in medicine: ZnO - a basis of powders, pastes, creams ( in DERMATOLOGY ) dental fillings (cements): Zn3(PO4)2 . 4 H2O "hopeit" Cadmium Cd toxic heavy metal metallothionein – strongly binds Cd2+ Cd intoxication kidney damage "Itai itai disease" - mass Cd poisoning in Japan in 1950 - the name comes from painful screams ( "itai" in Japanese = PAIN ) - the bones become soft and weak severe pain, fractures "Chemical castration" = destruction of seminiferous epithelium of testicles ( Zn2+ antagonism ) Mercury Hg (Hydrargyrum) Toxic effects 1) elemental Hg - very toxic when absorbed as a vapour through lungs - poorly absorbed through the gastrointestinal tract !!! (only purgative effect) 2) inorganic Hg compounds HgCl2 - "sublimate" - soluble in water = toxic - corrosive ulceration of GI tract - renal failure Hg2Cl2 – "calomel" - low solubility = less toxic - it was used in medicine ! (diuretic and purgative effect, ointments in dermatology) - calomel electrod (reference electrod - measurement of pH) 2) organic Hg compounds - often extremely toxic - dimethylmercury Hg(CH3)2 damage of CNS (central nervous system) - one of the strongest known neurotoxins ! "The Minamata Disease" - mass Hg poisoning in Japan in 1956 - Minamata Bay – waste industrial water with Hg2+ biomethylation by a variety of microorganism bioaccumulation in FISH dietary intake from fish diets Poison grain disaster in Iraq (1971) - seed grain mercury-treated to prevent rot was used as FOOD Elements of group III III A III B B Borum Sc Al Aluminium Y Ga La In Ac Tl Ra Boron B (Borum) PLANTS – micromineral Boric acid H3BO3 - a very weak acid - disinfectant (used in dermatology and ophtalmology) - also toxic properties ! glutamine synthetase glutamine NH3 + glutamate H3BO3 = inhibitor toxic to BRAIN Sodium tetraborate Na2B4O10 = borax (fusible glaze for pottery) Aluminium Al in human body – only traces – contamination ? Al(OH)3 together with MgO or Mg(OH)2 oral antacid (neutralization of acid in the stomach) Al – also considered as toxic ACID RAIN SOIL release of Al3+ ! Elements of group IV IV A IV B C Carboneum Ti Si Silicium Zr Ge Hf Sn Stannum Pb Plumbum Carbon C (Carboneum) the most important biogenic element organic compounds (covalent bonds C-C, chains, rings) biomolecules: proteins, nucleic acids, lipids, carbohydrates combustion CO, CO2 respiration CO2 Toxic: CO HCN competes with O2 for hemoglobin reaction with cytochromes inactivation of cell respiration Silicon Si (Silicium) abundant element in lithosfere, but not useful in human body SiO2 (silica) Quartz - common mineral in the Earth‘s crust - many varieties: amethyst (purple) citrine (yellow) morion (dark-brown) rose quartz (pink) - in many rocks (granit, sandstone), metallic ores cutting, breaking, crushing, ... inhalation of fine SiO2 dust SILICOSIS - lung disease occupational disease (miners, ceramics workers) progressive, signs of it will appear years after exposure ! Silicic acids: general formula n SiO2 . m H2O H4SiO4 orthosilicic acid Silicates - salts of silicic acids SiO44- Si atom - tetrahedral coordination by 4 oxygens complex structure - different degrees of "polymerization" linear arrangement FIBERS (asbestos) planar arrangement MICAS Silicate minerals - largest class of rock-forming minerals





