What's New in the Management of Pain in the ICU
advertisement

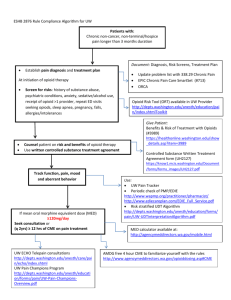
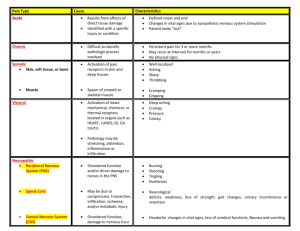
WHAT’S NEW IN THE MANAGEMENT OF PAIN IN THE ICU Mona K. Patel, PharmD Clinical Pharmacy Manager, Surgical ICU NewYork-Presbyterian Hospital Columbia University Medical Center October 2, 2015 DISCLOSURES None OBJECTIVES Explain the etiology of pain in critically ill patients Describe consequences of uncontrolled pain in critically ill patients Identify tools for the assessment of pain Outline methods for the management of pain INCIDENCE OF PAIN IN CRITICALLY ILL PATIENTS Leading cause of stress in critically ill patients Many patients will experience moderate to severe pain at rest and/or during procedures >50% of medical and surgical ICU patients experience moderate to severe pain at rest >50% recall moderate to extreme pain after ICU discharge Anesthesiology 2007;107:858-860. Anesthesiology 2007;106:687-695. Intensive Crit Care Nurs 2007;23:298-303. Crit Care Med 2008;36:2801-2809. NOCICEPTIVE PAIN Physiol Rev 2014;94:81-140. CAUSES OF PAIN Tracheal suctioning Turning Dressing changes Immobility Tubes, drains, catheters Surgical incisions Altered sensorium Pain Trauma Anesthesiology 2007;107:858-860. Am J Crit Care 2001;10:238-251. PROCEDURAL PAIN Common procedures can be a significant source of pain Procedure N (%) Pre-procedural pain intensity* Pain intensity during procedure* P value Wound drain removal 75 (1.6) 2 (0-4) 4.5 (2-7) <0.0001 Chest tube removal 292 (6.1) 2 (0-4) 5 (3-7) <0.0001 Arterial line insertion 199 (4.1) 1 (0-2.5) 4 (2-6) <0.0001 Endotracheal suctioning 767 (15.9) 1 (0-4) 4 (1-6) <0.0001 Peripheral blood draw 328 (6.8) 0.5 (0-3) 3 (1-5) <0.0001 Positioning 371 (7.7) 1 (0-4) 3 (0-5) <0.0001 * Data are shown as median (IQR) Am J Respir Crit Care Med 2014;189:39-47. LITTLE PROGRESS WITH ICU PAIN Routine aspects of ICU care are the most troublesome for patients 1990 63% remembered moderate to severe pain 2007 50% remembered unmet analgesic needs Heart Lung 1990;19:526-533. Intensive Crit Care Nurs 2007;23:298-303. LITTLE PROGRESS WITH ICU PAIN Pain is not being recognized and treated 842 ICU nurses surveyed • 33% used pain assessment tools for patients unable to communicate • 42% targeted treatment to pain score • 61% reported pain scores during nursing handoff Observational study including 1,381 ICU patients Day 2 (n=1,360) Day 4 (n=1,256) Day 6 (n=1,099) Analgesia Assessment Treatment 42% 90% 39% 80% 37% 74% Procedural pain Assessment Treatment 35% 22% 35% 21% 35% 22% Am J Crit Care 2012;21:251-259. Anesthesiology 2007;106:687-695. CONSEQUENCES OF PAIN Inadequate sleep Impairment of tissue perfusion Traumatic memories after ICU discharge Catabolic hypermetabolism Post traumatic stress disorder Difficulty managing severe pain Chronic pain Delirium Decreased quality of life Agitation Immune system impairment Crit Care Med 1998;26:651-659. Intensive Care Med 1979;5:89-92. Crit Care Clin 1999;15:167-184. Arch Surg 1991;126:338-342. RELATIONSHIP BETWEEN PAIN, AGITATION AND DELIRIUM N Engl J Med 2014;370:444- NECESSITY OF PAIN CONTROL Patient comfort Mobilization Judicious use of sedative agents Decrease length of mechanical ventilation Reduce ICU length of stay Crit Care Med 2006;34:1691-1699. Anesthesiology 2009;111:1308-1316. J Trauma Nurs 2011;18:52-60. Anesthesiology 2009;111:1308-1316. PAIN ASSESSMENT REDUCES SEDATIVE USE Day 2 Pain Assessment? P value No (n=631) Yes (n=513) Any sedative 86% 75% < 0.01 Midazolam 65% 57% < 0.01 Propofol 21% 17% 0.06 Other 6% 4% 0.03 Anesthesiology 2009;111:1308-1316. PAIN ASSESSMENT IMPROVES OUTCOMES Outcome Day 2 Pain Assessment? Unadjusted OR P value Adjusted OR P value No Yes ICU Mortality 22% 19% 0.91 0.69 1.06 0.71 ICU LOS 18 d 13 d 1.70 < 0.01 1.43 0.04 MV duration 11 d 8d 1.87 < 0.01 1.40 0.05 Ventilator Acquired Pneumonia 24% 16% 0.61 < 0.01 0.75 0.21 Anesthesiology 2009;111:1308-1316. ASSESSMENT OF PAIN Assess ≥ 4x/shift and before/after any procedure and analgesic administration Assessment scales should be used to determine if intervention needed and is adequate Patient self-report (gold standard) • Numeric Rating Scale (NRS) • Visual Analog Scale (VAS) Behavioral Pain Scale (BPS) Critical Care Pain Observation Tool (CPOT) Vital signs should not be used alone to assess pain Crit Care Med 2013;41:263-306. www.iculiberation.org BEHAVIORAL PAIN SCALE Item Description Score Facial expression Relaxed Partially tightened (e.g. brow lowering) Fully tightened (e.g. eyelid closing) Grimacing 1 2 3 4 Upper limbs No movement Partially bent Fully bent with finger flexion Permanently retracted 1 2 3 4 Compliance with ventilation Tolerating movement Coughing but tolerating ventilation for most of time Fighting ventilator Unable to control ventilation 1 2 3 4 Crit Care Med 2001;29:2258-2263. CRITICAL CARE PAIN OBSERVATIONAL TOOL Indicator Score Description Relaxed, neutral 0 No muscle tension observed Tense 1 Presence of frowning, brow lowering, orbit tightening and levator contraction or any other change (e.g. opening eyes or tearing during nociceptive procedures) Grimacing 2 All previous facial movements plus eyelid tightly closed (the patient may present with mouth open or biting the endotracheal tube) Absence of movements or normal position 0 Does not move at all (doesn’t necessarily mean absence of pain) or normal position (movements not aimed toward the pain site or not made for the purpose of protection) Protection 1 Slow, cautious movements, touching or rubbing the pain site, seeking attention through movements Restlessness/Agitation 2 Pulling tube, attempting to sit up, moving limbs/thrashing, not following commands, striking at staff, trying to climb out of bed Tolerating ventilator or movement 0 Alarms not activated, easy ventilation Compliance with the ventilator (intubated patients) Coughing but tolerating 1 Coughing, alarms may be activated but stop spontaneously Fighting ventilator 2 Asynchrony: blocking ventilation, alarms frequently activated OR Talking in normal tone or no sound 0 Talking in normal tone or no sound Sighing, moaning 1 Sighing, moaning Crying out, sobbing 2 Crying out, sobbing Relaxed 0 No resistance to passive movements Tense, rigid 1 Resistance to passive movements Very tense or rigid 2 Strong resistance to passive movements or incapacity to complete them Facial expressions Body movements Vocalization (extubated patients) Muscle tension Am J Crit Care 2006;15:420-427. TREATMENT OF PAIN Risk assessment • • • • Severity of illness Chronic pain conditions Coexisting symptoms Frequency and invasiveness of therapies Early recognition • Use of validated tools • Frequent assessment Nonpharmacologic • Non-pharmacologic (e.g. music therapy, relaxation technique) • Positioning • Removal of offending agent Pharmacologic • Non-opioids • Opioids Chest 2009;135:1069-1074. Q1: WHICH OF FOLLOWING ARE BENEFITS OF PAIN ASSESSMENT? A. Reduce incidence of ileus B. Decrease use of sedation C. Increase length of mechanical ventilation D. Decrease incidence of fractures E. Improvement in urine output Q2: ROUTINE PROCEDURES DO NOT CAUSE PAIN IN CRITICALLY ILL PATIENTS A. True B. False OPIOIDS FOR THE TREATMENT OF PAIN First line for the treatment of non-neuropathic pain Choice of agent is multi-factorial Onset of action Duration of action Elimination Adverse effects Cost Crit Care Med 2013;41:263-306. OPIOIDS FOR THE TREATMENT OF PAIN Opiate IV potency ratio (relative to morphine) Onset (IV) T1/2 (h) Fentanyl 100:1 1-2 min 2-4 No Demethylation, CYP3A4 substrate Accumulation with hepatic impairment Hydromorphone 5:1 5-15 min 2-3 No Glucuronidation Accumulation with hepatic/renal impairment Accumulation with hepatic/renal impairment; histamine release; active metabolite Use ideal body weight for obese patients; cost; glycine neurotoxicity Active metabolite Metabolism Morphine 1:1 5-10 min 1.5-5 Yes Demethylation, glucuronidation Remifentanil 20:1 1-3 min 0.05 No Hydrolysis by plasma esterases Clinical considerations Crit Care Med 2013;41:263-306. AJHP 2015;72:1531-1543. Chest 2009;135:1075-1086. Chest 2008;133:552-565. Crit Care Clin 2009;25:431-449. MAXIMIZING OPIOID EFFECTIVENESS Weigh risks vs benefits when determining dose or duration Use caution when determination of equipotent doses Consider all opioids equally effective at equipotent doses Aggressively treat patients with severe pain Incorporate non-opioids Chest 2009;135:1075-1086. NON-OPIOID MANAGEMENT OF PAIN Gabapentin (neuropathic pain) Carbamazepine (neuropathic pain) Ketamine Acetaminophen Local and regional anesthetics Nonsteroidal anti-inflammatory drugs (NSAIDS) BENEFITS OF MUTLI-MODAL PAIN REGIMENS Decrease opioid administration Decrease incidence of opioid related adverse effects Reduce severity of opioid related adverse effects Achieve better neuropathic pain control Crit Care Med 2013;41:263-306. Anesth Analg 2002;95:1719-1723. Anesth Analg 2005;101:220-225. CHOOSING THE RIGHT PAIN REGIMEN Dependent on many factors Drug pharmacokinetics Active metabolites Frequency, severity of pain Presence of contraindications Mental status Unknown drug-nutrient interactions Gastrointestinal absorption Restricted personnel for administration Source of pain Intact patient cognition for administration Chronic pain Possible adverse effects of agents Pain should be treated first regardless of chosen regimen Crit Care Med 2013;41:263-306. Chest 2009;135:1075-1086. ANALGESIA-FIRST SEDATION “Analgosedation” or “A-1” Treat pain and discomfort first Add sedatives, if needed, after treating pain Crit Care Med 2013;41:263-306. Chest 2009;135:1075-1086. Chest 2008;133:552-565. WHY DO WE CARE ABOUT ANALGOSEDATION? Sedation is commonly used to manage patient discomfort Sedatives are associated with adverse drug effects Deep sedation is harmful when not indicated Pain control will help improve comfort and reduce sedation requirements Ann Pharmacother 2012;46:530-540. A PROTOCOL OF LIMITED SEDATION FOR CRITICALLY ILL PATIENTS Mechanically ventilated medical and surgical patients No sedation (n=55) Sedation (n=58) prn IV morphine prn IV morphine + propofol infusion x 48h, then prn morphine + IV midazolam infusion Daily interruption of sedation Goal Ramsey 3-4 Continued discomfort Nonpharmacologic prn IV haloperidol Propofol infusion x6h Lancet 2010;375:475-480. A PROTOCOL OF LIMITED SEDATION FOR CRITICALLY ILL PATIENTS Greater days without ventilation in no sedation group Mean difference 4.2 days (95% CI 0.3-8.1), p=0.019 Sedation increased length of stay ICU: HR 1.86 (95% CI 1.1-3.2), p=0.032 Hospital: HR 3.57 (95% CI 1.5-9.1), p=0.004 No difference in mortality between no sedation vs sedation groups ICU: 22% vs 38%, p=0.06 Hospital: 36% vs 47%, p=0.27 Great incidence of delirium in no sedation group Lancet 2010;375:475-480. REMIFENTANIL ANALGOSEDATION Randomized, multicenter study with mechanically ventilated MICU and SICU patients Remifentanil infusion +/- propofol infusion (n=96) vs morphine or fentanyl infusion + propofol, midazolam, or lorazepam infusion (n=109) Patients in remifentanil group more likely to be extubated on day 1-3 • OR 1.86 (95% CI 1.11-3.11), p=0.02 No difference in ICU discharge in remifentanil group on day 1-3 • OR 1.89 (95% CI 1.00-3.59), p=0.05 Intensive Care Med 2009;35:291-298. REMIFENTANIL ANALGOSEDATION Randomized, multicenter study with mechanically ventilated MICU and SICU patients Remifentanil infusion +/- midazolam bolus (n=57) vs morphine or fentanyl infusion + midazolam infusion or bolus (n=48) Patients in remifentanil group had shorter time to extubation • -53.5h (95% CI -111.4 to 4.4), p=0.033 No difference in ICU discharge • -22.5h (95% CI -201.5 to 156.5), p=0.326 Crit Care 2005;9:R200-R210. ADVANTAGES OF ANALGOSEDATION Reduce sedative use Lower risk of prolonged sedative effects Decrease risk for sedative-related adverse effects Shorten mechanical ventilation time Reduce length of stay Ann Pharmacother 2012;46:530-540. DISADVANTAGES OF ANALGOSEDATION Opioid associated delirium Increased opioid requirement Unpleasant recall Reduced gastrointestinal motility Immunosuppression Long term outcomes unknown Opioid withdrawal Hyperalgesia Ann Pharmacother 2012;46:530-540. ROLE OF ANALGOSEDATION IN CRITICALLY ILL PATIENTS May be considered prior to initiation of sedation in critically ill patients Addition of non-opioid therapy may minimize some disadvantages Avoid in certain patient populations Paralysis Status epilepticus Withdrawal syndromes Elevated intracranial pressures KETAMINE Phencyclidine derivative Commonly used for procedural sedation, intubation, spinal analgesia and postoperative pain management Antagonism of glutamate at N-methyl-D-aspartate (NMDA) receptor; activation of μ, κ, δ receptors; monoaminergic, muscarinic, nicotinic receptor antagonism Inhibition of “wind-up phenomenon” Minerva Anestesiol 2011;77:812-820. J Palliat Care 2012;15:474-483. KETAMINE Labeled indication: induction and maintenance of general anesthesia Induction: (IV) 1-4.5 mg/kg (IM) 16.5-13 mg/kg Maintenance of anesthesia: (IV) 15-90 mcg/kg/min Studied regimens for pain control Oral Epidural IV - PCA, continuous infusion, single dose Continuous IV infusion of subanesthetic ketamine may have a growing role for the management of pain in ICU patients J Palliat Med 2012;15:474-483. Anesth Analg 2004;99:482-495. REDUCTION OF OPIOID USE WITH KETAMINE Patient population Cardiac surgery Intervention KET 75 mcg/kg bolus then 1.25 mcg/kg/min (n=44) vs PL (n=46) x48h Narcotic reduction Oxycodone requirements: KET 103±44 mg vs PL 125±45 mg, p=0.023 Knee arthroplasty KET 0.5 mg/kg then 3 mcg/kg/min during surgery then 1.5 mcg/kg/min (n=20) vs PL (n=20) x48h Morphine requirements: KET 45±20 mg vs PL 69±30 mg, p<0.02 Major abdominal surgery KET 0.5 mg/kg then 2 mcg/kg/min x 24h then 1 mcg/kg/min x24h (n=41) vs PL (n=52) Morphine requirements: KET 58±35 mg vs PL 80±37 mg, p<0.05 KET=ketamine; PL=placebo Pain scores Adverse effects No difference No difference No difference No difference 4 patients with psychomimetic effects with KET vs 0 with PL No difference No difference Anesth Analg 2004;99:1295-1301. Anesth Analg 2005;100:475-480. Anesth Analg 2003;97:843-847. REDUCTION OF OPIOID USE WITH KETAMINE Reduction in morphine use after major abdominal surgery Perioperative (n=23) 0.5 mg/kg bolus then 2 mcg/kg/min x 48h starting intraoperatively Intraoperative (n=27) 0.5 mg/kg bolus then 2 mcg/kg/min intraoperatively only Placebo (n=27) -- Less morphine use in perioperative group • Perioperative 27 mg vs intraoperative 48 mg vs placebo 50 mg, p=0.008 Better pain scores in perioperative and intraoperative groups vs placebo at H4 (p=0.004), H24 (p=0.0001), H48 (p=0.001) Anesth Analg 2008;106:1856-1861. REDUCTION OF OPIOID USE WITH KETAMINE Reduction in morphine use after thoracotomy PCA with morphine 1.5 mg + placebo (n=20) vs PCA morphine 1 mg + ketamine 5 mg (n=21) x 4h Ketamine group required 45% less morphine over 4h (p<0.001) • Hour 1: 6.8±1.9 mg vs 3.7±1.2 mg, p=0.001 • Hour 2: 5.5±3.6 mg vs 2.8±2.3 mg, p=0.008 Lower maximal pain scores in ketamine group • 5.6±1.0 vs 3.7±0.7, p=0.001 No difference in adverse effects Chest 2009;136:245-252. ADVANTAGES OF KETAMINE Bronchodilation Preservation of cardiac output Not associated with bradycardia or hypotension Low side effect profile at subanesthetic doses No depression of respiratory drive Decrease in opioid tolerance IV administration Low cost DISADVANTAGES OF KETAMINE Adverse effects Hypersalivation Hypertension Nystagmus Tachycardia Psychomimetic effects Negative inotrope in heart failure or cardiogenic shock states Unclear safety in patients with neurological injury, pulmonary hypertension, cardiac ischemia Unknown impact on delirium Optimal dosing not known IV ACETAMINOPHEN – WHAT DO WE KNOW? Approved in 2010 Opioid sparing effects seen in many patient populations with once or repeat dosing Total hip or knee replacement Major abdominal or pelvic surgery Abdominal laparoscopy Molar surgery Abdominal hysterectomy Tosillectomy Well tolerated and safe Pharmacotherapy 2014;34:34S-39S. ACETAMINOPHEN PHARMACOKINETICS Single dose pharmacokinetics of IV vs oral vs rectal Pain Pract 2012;12:523-532. REDUCTION OF OPIOID USE WITH IV ACETAMINOPHEN Patient population and design Total abdominal hysterectomies Retrospective Bariatric surgery Retrospective Surgical knee procedures Retrospective, case-control Intervention IVA with opioids (n=50) vs OA (n=50) IVA with opioids (n=38) vs OA (n=47) IVA with opioids (n=25) vs OA (n=75) IVA=IV acetaminophen OA=opioids only Morphine equivalents Pain scores during intervention Length of stay Not assessed Not assessed Not assessed Not assessed Not assessed No difference Post-operative day 1-2 IVA 47±24 mg vs OA 68±37 mg, p=0.003 Total perioperative period IVA 73±24 mg vs OA 99±39 mg, p=0.001 Postoperative day 1 IVA 100 mg vs OA 165 mg, p=0.018 Total postoperative course IVA 135 mg vs OA 113 mg, p=0.987 Daily IVA 45 mg vs OA 38 mg, p=0.845 Pharmacotherapy 2014;34:27S-33S. J Surg Res 2015;195:99-104. Pharmacotherapy 2014;34:22S-26S. IV VERSUS PO ACETAMINOPHEN Limited opioid sparing with IV vs PO acetaminophen in cardiac surgery patients 1g po q6h (n=38) vs 1g IV q6h (n=39) until morning after surgery Lower ketobemidone with IV acetaminophen • 17.4±7.9 mg vs 22.1±8.6 mg (p=0.016) No difference in pain scores No difference in visual analog scores >3 No difference in nausea/vomiting J Cardiothorac Vasc Anesth 2005;19:306-309. ADVANTAGES OF IV ACETAMINOPHEN Faster onset of action compared to other routes Reduction of opioid requirements Well tolerated in clinical trials Administration in patients unable to tolerate alternate routes DISADVANTAGES OF IV ACETAMINOPHEN Administration considerations Stable for maximum 6h after vial is opened Fluid restricted patients Single use vials Pregnant patients Limited evidence comparing to oral and rectal routes Unknown impact on clinical outcomes Cost LIPOSOMAL BUPIVACAINE – WHAT DO WE KNOW THUS FAR? Approved in 2011 Local anesthetic for use in management of postsurgical pain in adults Use of DepoFoam technology releases bupivacaine over extended period of time offering lasting pain control without affecting the active ingredient Exparel (bupivacaine liposomal injectable suspension) [package insert] Pacira Pharmaceuticals, Inc.;2014. LIPOSOMAL BUPIVACAINE – WHAT DO WE KNOW THUS FAR? Studied in many patient populations Inguinal hernia repair Total knee arthroplasty Hemorrhoidectomy Breast augmentation Bunionectomy Open colectomy Ileostomy reversal Abdominal hernia repair Robotic prostatectomy Narcotic reduction and better pain scores noted vs placebo Inconsistent benefit when compared to conventional bupivacaine J Clin Ther 2015;37:1354-1371. LIPOSOMAL BUPIVACAINE FOR POSTSURGICAL ANALGESIA Equivalent randomized, controlled trials Study Intervention Results Laparoscopic urologic surgery patients 0.25% bupivacaine (weight based) (n=64) vs LB 266 mg (60 mL) (n=68) • No difference in median total opioid dose, pain score, length of hospital stay, time to first opioid use (p>0.05) Total knee arthroplasty Bupivacaine 150 mg (60 mL)(n=53) vs LB 266 mg (20 mL) + bupivacaine 75 mg (30 mL) (n=58) • No difference in pain scores assessed on morning or afternoon on days 1, 2, 3, hospital length of stay, knee range of motion, opioid use, nausea (p>0.05) LB 266 mg (60 mL) (n=40) vs 0.5% ropivacaine + 1:200,000 epinephrine + 1% tetracaine 30 mg (40 mL) (n=40) • No difference in total pain score, passive extension, nausea, vomiting, opioid consumption, ambulation (p>0.05) • Higher mean pain score with LB on POD 0 (3.84 vs 2.91, p<0.05) • Lower flexion in with LB (94° vs 101°, p=0.001) • Higher opioid consumption with LB on POD 0 (25.5 mg vs 13.9 mg, p<0.05) and lower with LB on POD 1 (3.9 mg vs 9.1 mg, p<0.05) Periarticular administration Total knee arthroplasty LB=liposomal bupivacaine J Athroplasty 2015;30:64-67. J Athroplasty 2015;30:325-329. J Endourol 2015;29:1019-1024. LIPOSOMAL BUPIVACAINE FOR POSTSURGICAL ANALGESIA Randomized, controlled trial in robotic assisted hysterectomy patients LB 13 mg (30 mL) (n=28) vs control 0.25% bupivacaine + 1:200,000 epinephrine (30 mL) (n=30) Lower total pain scores and opioid use with LB T0-24h (LB vs control) T24-48h (LB vs control) T48-72h (LB vs control) Maximum pain score 5 (0-10) vs 7 (0-10), p=0.01 4 (0-8) vs 5 (1-10), p=0.04 3 (0-8) vs 5 (0-10), p=0.047 Opioid use 13 (0-50) vs 25 (5-88), p=0.02 3 (0-27) vs 8 (0-68), p=0.02 2 (0-12) vs 5 (0-40), p=0.30 Less nausea with LB (25% vs 57%, p=0.01) No difference in hospital length of stay with LB vs control • 11±9h vs 17±14h, p=0.06 LB=liposomal bupivacaine Gynecol Oncol 2015;138:609-613. LIPOSOMAL BUPIVACAINE FOR POSTSURGICAL ANALGESIA Positive, randomized controlled trial in open total hysterectomy patients 0.5% bupivacaine (40 mL) (n=30) vs LB 266 mg (60 mL) (n=30) Marginal benefits in opioid consumption with LB • Morphine use T0-24h: 47.7 (28.8) mg vs 33.6 (24.3) mg, p=0.05 • Hydrocodone 5mg + acetaminophen 325 mg use T24-48h: 3.6 (2.8) vs 1.9 (1.7), p=0.01 * p<0.01 Lower pain scores with LB ** p<0.001 Anesth Analg 2015 [Epub ahead of print] ADVANTAGES OF LIPOSOMAL BUPIVACAINE May reduce opioid requirements Decreased need for patient controlled analgesia pumps Convenience Good safety profile DISADVANTAGES OF LIPOSOMAL BUPIVACAINE Not studied in critically ill patients No role in non-surgical patients Unknown impact in chronic pain patients Safety in pregnant patients unknown Conflicting literature Cost NSAIDS Relieve inflammation and associated nociceptive stimuli via inhibition of cylooxygenase Agents available Ibuprofen (IV, po, topical) Ketorolac (IV, po, nasal, ophthalmic) Diclofenac (IV, po, topical, ophthalmic) Lancet 2011;377:2215-2225. IV DICLOFENAC FDA approval in December 2014 Significant improvement in pain intensity and opioid reduction compared to placebo Limited data comparing to other NSAIDS Anesth Analg 2012;115:1212-1220. IV DICLOFENAC Randomized controlled trial in abdominal or pelvic surgery patients IV diclofenac (n=173) vs ketorolac (n=82) vs placebo (n=76) Significant analgesia with IV diclofenac vs placebo IV diclofenac vs IV ketorolac • No difference in sum of pain intensity difference, median time to >30% pain intensity reduction, opioid requirements, total pain relief • No difference in adverse effects Anesth Analg 2012;115:1212-1220. IV DICLOFENAC Randomized controlled trial in orthopedic surgery patients IV diclofenac (n=145) vs ketorolac (n=60) vs placebo (n=72) Significant analgesia with IV diclofenac vs placebo IV diclofenac vs IV ketorolac Less morphine used in patients receiving diclofenac than ketorolac • 11.8 mg vs 18.1 mg, p=0.008 Less severe pain (VAS≥70 mm) with diclofenac than ketorolac • 42.1% vs 51.7%, p≤0.05 Incidence of adverse effects were similar Clin J Pain 2013;29:655-663. ADVANTAGES OF NSAIDS Several products available Administration to patients unable to tolerate oral agents Reduction in opiate use and better pain control Targeting pain secondary to inflammation DISADVANTAGES OF NSAIDS Role of NSAIDS is limited for pain control in ICU patients Increased risk for bleeding and renal dysfunction FDA warning for increased risk of heart attack, heart failure and stroke in patients with or without heart disease or risk factors for heart disease Increased risk with higher doses, longer duration Animal and in vitro data showing impaired bone healing http://www.fda.gov/Drugs/DrugSafety/ucm451800.htmCurr Opin. Rheumatol 2013;25:524-531. The ultimate question: How can patient comfort be safely and reliably achieved in the ICU? PAIN PROTOCOL Systematic assessment, treatment and prevention is necessary Pain protocols are associated with positive outcomes Decrease use of psychoactive medications Reduce medication induced coma Decrease pain and agitation Reduce ICU length of stay Shorten duration of mechanical ventilation Incorporate multi-modal therapy Crit Care Med 2006;34:16911699. Anesth Analg 2010;111:451-463. ANALGESIA/SEDATION ALGORITHM 1. In Pain? Analgosedation? Ketamine? Acetaminophen? NSAIDS? Yes No Reassess often Yes Analgesia may be adequate to reach RASS target No Under sedated • Propofol 5-30 mcg/kg/min • Dexmedetomidine 0.2-1.5 mcg/kg/hr (if delirious/weaning) • Midazolam 1-3 mg prn (alcohol withdrawal or propofol intolerance) CAM-ICU negative Reassess q6-12h RASS=Richmond Agitation Sedation Scale SAT=Spontaneous awakening trial SBT=Spontaneous breathing trial CAM-ICU=Confusion Assessment Method for the ICU 2. At RASS target? Yes Reassess often Bolus dosing prn with either • Fentanyl 50-100 mcg • Hydromorphone 0.1-0.3 mg • Morphine 2-5 mg Controlled or anticipated control with <3 bolus/doses/h No • Fentanyl 50-300 mcg/h infusion • Fentanyl 25-100 mcg prn pain No Over sedated Hold sedative/analgesic to achieve RASS target. Restart at 50% if clinically indicated SAT+SBT daily physical therapy 3. Delirium? CAM-ICU positive • Non-pharmacological management • Pharmacological management www.icudelirium.org Assess using validated scales ≥4x/shift, before/after any procedure and analgesic administration Pain Analgosedation (assess for contraindications) Non-pharmacologic Pharmacologic (initiate as adjunct when allowable) • • • • Music Relaxation Positioning Remove offending agent Non-opioid (initiate as adjunct when allowable) Ketamine • Caution in patients with heart failure, cardiogenic shock, neurological injury, pulmonary hypertension, cardiac ischemia Acetaminophen • Route of administration dependent on access and absorption • Avoid in patients with liver dysfunction Opioid (first line for non-neuropathic pain) NSAIDS • Route of administration dependent on access and absorption • Avoid in patients with or at risk for bleeding, renal dysfunction Fentanyl • Caution in patients with liver dysfunction • Intermittent dosing may be considered first before continuous infusion Morphine • Caution in patients with liver and renal dysfunction • Consider histamine release especially in hypotensive patients Local anesthetics • Surgical pain only • Local, regional administration Hydromorphone • Caution in patients with liver and renal dysfunction • Intermittent dosing may be considered first before continuous infusion Remifentanil • Continuous infusion should be used for sustained pain relief vs bolus dosing • Rapid cessation of analgesia with therapy discontinuation Gabapentin, Carbamazepine • Neuropathic pain PAIN METRICS Assess Treat • Percentage of time patients are monitored for pain ≥4x/shift • Percentage of time patients are in significant pain • Compliance with use of ICU pain scoring systems • Percentage of time pain is treated within 30 min of detecting significant pain Prevent • Percentage of time patients receive preprocedural analgesics and/or nonpharmacological interventions • Percentage compliance with institutional/ICU pain protocol www.iculiberation.org. Crit Care Med 2013;41:263-306. ABCDEF BUNDLE Goal is to improve pain management and reduce delirium and long-term consequences Symptoms Pain Monitoring • • • CPOT NRS BPS • Richmond Agitation-Sedation Scale (RASS) Sedation-Agitation Scale (SAS) Agitation • • Delirium • Confusion assessment method for intensive care unit (CAM-ICU) Intensive care delirium screening checklist (ICDSC) Care A: Assess, prevent and manage pain B: Both spontaneous awakening trials (SAT) and spontaneous breathing trials (SBT) C: Choice of analgesia and sedation D: Delirium: assess, prevent and manage E: Early mobility and exercise F: Family engagement and empowerment www.iculiberation.org. Crit Care Med 2013;41:263-306. Regular rounding Collaborate within multidisciplinary team Avoid drug- drug, drug-food, drugdisease interactions Educate others Pharmacist’s role in ABCDEF Conduct medication reconciliation Identify methods to minimize costs Assist with dosing therapy Assist with development and compliance with hospital protocols or guidelines Ensure appropriate monitoring PAIN MANAGEMENT IN CRITICALLY ILL PATIENTS Uncontrolled pain is common in critically ill patients and is associated with numerous consequences Pain is under assessed and under recognized Multi-modal therapy, non-pharmacologic and pharmacologic, should be considered Treat pain first before use of sedatives Bundles addressing pain control should be implemented Q3: WHICH OF THE FOLLOWING ARE CONSEQUENCES OF UNCONTROLLED PAIN? A. Post traumatic stress disorder B. Sleep disorders C. Delirium D. Agitation E. All of the above Q4: WHICH OF THE FOLLOWING ARE BENEFITS OF ANALGOSEDATION? A. Reduced pressure ulcers B. Increased urine output C. Decreased length of mechanical ventilation D. Greater muscle mass E. None of the above QUESTIONS? WHAT’S NEW IN THE MANAGEMENT OF PAIN IN THE ICU Mona K. Patel, PharmD Clinical Pharmacy Manager, Surgical ICU NewYork-Presbyterian Hospital Columbia University Medical Center October 2, 2015