Chapter 4 Test - Atomic Structure Key
advertisement
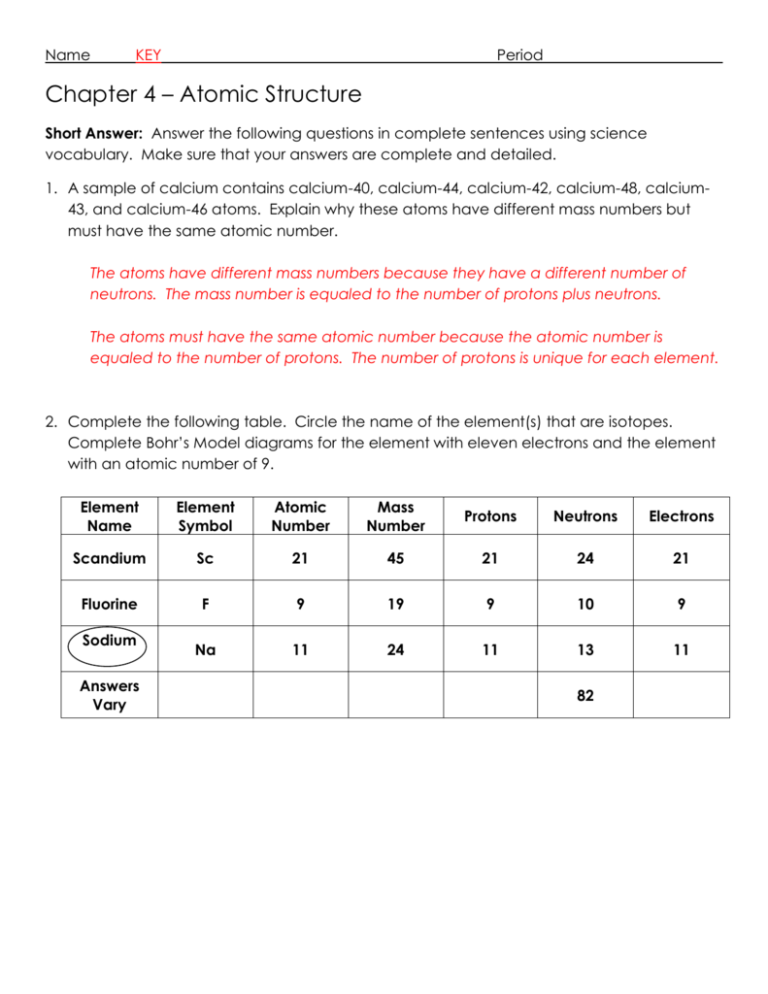
Name KEY Period Chapter 4 – Atomic Structure Short Answer: Answer the following questions in complete sentences using science vocabulary. Make sure that your answers are complete and detailed. 1. A sample of calcium contains calcium-40, calcium-44, calcium-42, calcium-48, calcium43, and calcium-46 atoms. Explain why these atoms have different mass numbers but must have the same atomic number. The atoms have different mass numbers because they have a different number of neutrons. The mass number is equaled to the number of protons plus neutrons. The atoms must have the same atomic number because the atomic number is equaled to the number of protons. The number of protons is unique for each element. 2. Complete the following table. Circle the name of the element(s) that are isotopes. Complete Bohr’s Model diagrams for the element with eleven electrons and the element with an atomic number of 9. Element Name Element Symbol Atomic Number Mass Number Protons Neutrons Electrons Scandium Sc 21 45 21 24 21 Fluorine F 9 19 9 10 9 Na 11 24 11 13 11 Sodium Answers Vary 82 3. What is the difference between the atomic number of an atom and its mass number? The atomic number is the number of protons in an element. Since the number of electrons is the same as the number of protons, the atomic number also equals the number of electrons. The mass number is equaled to the number of protons plus neutrons. 4. Explain why a neutral atom cannot have one proton, one neutron, and two electrons? Protons have a positive charge (+) and electrons have a negative charge (-). Neutrons have no charge. Therefore, a neutral atom cannot have more electrons than protons, as it would have an overall negative charge (-1). 5. The nucleus of an atom contains six neutrons and six protons. The nucleus of a second atom contains six neutrons and five protons. Are they atoms of different elements or isotopes of the same element? Explain you answer. The atoms are different elements, since they have different numbers of protons. The first atom has six protons and is therefore carbon. The second atom has five protons and is therefore boron. 6. Brightly colored neon lights consist of tubes filled with a gas. When an electric current passes through the tubes, different colors are emitted. Why might you conclude that the tubes in a multicolored display contain more than one element? From the flame test lab, we discovered that different elements and/or compounds emit different colors of light. Therefore, if the “neon” light emitted different colored lights, we can conclude that the lights contain different elements and/or compounds.




