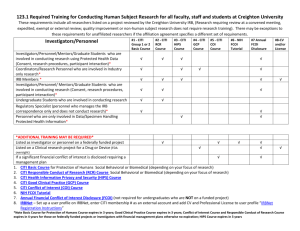
The Ethical Treatment of Human and Non-Human Subjects in
advertisement

Promoting Integrity in Your Laboratory Animal Welfare Research Program The CITI Program Paul Braunschweiger PhD Chair, University of Miami IACUC CITI Program Co-Founder University of Miami Miller School of Medicine Foundations for the Ethical Treatment of Animals in research • Aristotle – – – – Student of Plato, Teacher to Alexander the Great. Research - Empirical observation and experience. Scientific method. Animals: • Sensation, Passion, memory, understand relationships. • NOT Capable of Thought or reasoning – Aristotle believed that creatures were arranged in a graded scale of perfection. Plato and Aristotle – “The Athens School” by Raphael – Man at a higher state of perfection because Man can reason and reflect. – Animals and plants exist for man’s use, but not abuse. Foundations for the Ethical Treatment of Animals in Research • Ethical basis for animal welfare – 18th Century - Jeremy Bentham 1748 - 1832 • Utilitarianism, The moral status of animals • Animals had moral status and rights to be treated humanely. • “Cruelty towards animals is an incentive to cruelty towards men.” • In response to Descartes: “The question is not, Can they reason? nor, Can they talk? but, Can they suffer?” 1748 - 1832 Foundations for the Ethical Treatment of Animals in Research • Marshall Hall (1790–1857), a physician and noted physiologist, supported animal research but stated: • ‘Unhappily… the subjects of animal physiology are sentient, and every experiment is attended by pain and suffering.’ • 5 guiding principles of animal research: 1790–1857 – – – – – i) the lack of an alternative ii) a clear objective iii) the avoidance of repetition of work iv) the need to minimize suffering v) full and detailed publication of the results. In 1831, he outlined five principles to govern animal experimentation. Of the Principles of Investigation in Physiology. Lancet 1 (1856), pp. 393–394. The Principles of Humane Experimental Technique Russell WMS and Burch RL (1959) "The Principles of Humane Experimental Technique" – 3Rs – Reduction – Replacement – Refinement Foundations for the Ethical Treatment of Animals in Resarch • The Regulatory Requirements – 20th Century – Organizations, Codes and Legislation • Nuremburg Code – Point 3 • AALAS -1963 • The Guide to the Care and Use of Laboratory Animals (NRC) – 1963, 1996, 2010 RCR 12-29-07 Betrayal of the Public Trust Brings Regulation • 1984 Univ. Penn. Head Trauma Study. – PETA and ALF alleges inhumane research practices. – Videos – NIH investigated. Research activities not conducted according the existing policies. • • • • Lack of adequate anesthesia and analgesia. Lack of sterile technique. Lack of supervision by PI. Lack of training of laboratory personnel. – NIH Shut down the project. • Research Project permanently shut down. RCR 12-29-07 Outcome Animal Welfare Act – USDA / APHIS Public Law 99-198, December 23, 1985, Food Security Act of 1985. Subtitle F - Animal Welfare HREA & AWA -1985 • Grounded in the 3Rs and the Guide. Created: – Institutional Official – Attending Veterinarian – Creation of the IACUC • Composition. • Responsibilities. – – – – – Oversee all teaching and research activities with animals. Minimize pain and distress Education program. Inspect facilities. Investigate concerns • Appointed by CEO • Manage the potential conflicts of interest. Conflict of Interest (CoI) • A conflict of interest is a situation where financial or other personal considerations can compromise or bias one’s professional judgment and/or objectivity. • A potential conflict of Interest is a situation where a reasonable person would think that the professional's judgment is likely to be compromised. • A conflict of interest implies only some potential for bias, not a likelihood. • Potential Conflicts should be managed to prevent unethical behavior. Understanding and Managing the Conflict of Interest who are the stake holders? • The Public’s Interests – Society demands medical advances. – Society understands that animal subjects: • Are necessary before a clinical trial. (Nuremberg #3) • Cannot understand the research. • Cannot consent to participate in what may cause them harm, pain or death. – Society demands documentation that animal research is ethically designed and conducted. Understanding and Managing the Conflict of Interest who are the stakeholders? • • The investigator Interests – Get the data, publish the papers, get the grants, support their labs, get promoted and get a raise. • The Interests of the Veterinarians – Animal welfare / compliance Vs. Helping investigators get their work done. Well funded investigators can bring significant pressure to bear on the veterinary staff. • Institutional interests – Institutions want the grants, patents and indirect costs. The IACUC specifically empowered by the Federal Regulations and UM Policy to manage the tensions and conflicts. Understanding and Managing the Conflict of Interest Who manages the conflicting interests? • The Public’s Interests The IACUC specifically empowered by the Federal Regulations and institutional Policies to manage the tensions and conflicts between the stakeholders. • The investigator Interests • The Interests of the Veterinarians • Institutional interests The IACUC and Conflict Management • IACUC has the oversight responsibility for all animal use at the University. – Investigates instances of non-compliance. • Can suspend activities that are not being conducted ethically and put animals at risk. – Reports the good, the bad and ugly to: • Institutional Official • OLAW and USDA • IACUC appointed by highest institutional authority. – Protect the University’s interests – Manage the CoI – Maintain the Public’s trust Consequences of betrayal of the Public Trust • • • • • • • • • Puts subjects at risk Federal inquiry / intervention Institutional embarrassment Fines Wasted resources Personal embarrassment Loss of funding, Loss of livelihood Jail Justification and / or vindication for groups with anti-research agenda. A.L.F. RCR 12-29-07 Animal Rights • The movement seeks to: – End the rigid moral and legal distinction drawn between human and non-human beings. – End the status of animals as property. – End to their use in the research, food, clothing. – End use in the entertainment industries. • Bull fighting • Movies • Commercials Increase Integrity in Your Animal Care and Use Program Increasing the integrity of your animal care and use program. • Service oriented IACUC. – Think of the investigator as your client. – Review your process make it easy for investigators to comply. • Review the performance of your IACUC members. • Find an alternate for each regular IACUC member. Use the alternates in the protocol review process. Increasing the integrity of your animal care and use program. • Use the designated review process for low risk protocols and amendments. AAALAC • Post ApprovalAccreditation Protocol Monitoring (PAM) • Implement a rigorous education program for investigators, staff and students. – Web based, classroom, wet labs CITI Collaborative Institutional Training Initiative Educational opportunities CITI Program www.citiprogram.org CITI Program Founders CITI Program is a web based bioethics education program designed to promote the responsible conduct of research. March-2000 Karen Hansen, Fred Hutchinson Cancer Research Center, Seattle, WA., USA Paul Braunschweiger Ph.D. University of Miami Miami, Fl. USA www.citiprogram.org CITI – Program 4-2010 Participating Institutions and Organizations (~1310) CITI Developer Group (~70) Biosafety RCR CITI Executive Advisory Committee Founders L.A.W. CITI Editorial Board (15) Intl. HSRP HIPS GCP www.citiprogram.org CITI Administration – Office of Research Education, University of Miami CITI Program Milestones • • • • 10 Participating Institutions in 2000. New Courses added 2004. ~ 1140 Participating Institutions in 3- 2010. ~ 30,000 Learners per month. 40- 60% students • >360,000 Learners since 3-1-2009. • >1.4 million people have completed a course since 9-1-2000. • Subscriber Profile – Universities, colleges, medical centers, community hospitals, societies, government, commercial IRBs, industry CITI Program Milestones • International Initiatives – CITI - Collaborative International Training Initiative • Multi-language Course Site. 11-2007 updated 2-2009 – Spanish – Portuguese – Chinese, Japanese, Russian, Mongolian, French, Thai CITI Program Courses • Human Subject Protection – Good Clinical Practice (15) – Refresher Courses • Health Information Privacy and Security (HIPS) – Basic – Refresher • The Responsible Conduct of Research. – 5 Disciplines – Text, cases, quizzes. • Laboratory Animal Welfare (L.A.W.) – Basic – Refresher CITI Lab Animal Welfare Program • 10-2007 – CITI Lab Animal Welfare Working Group. – Michael Mann Ph.D. working group Leader • 2004 - Collaboration between CITI and Dr, M. Fallon at the Atlanta, VA. • 2008 - 2009 - Comprehensive review and updating • 2007 VA decided to close the of the–course content. www.ResearchTraining.org LAW course site. •• 5-2007 CITI offered the Collaborating CITI Version of RT.org to 2009 CITI International Centers. our participating institutions. • 2010 – New Case Based Refresher Course IACUC members – Working with Fish in The Field and in The Laboratory. RCR 12-29-07 March 28, 2008 CITI Lab Animal Working Group Mike Mann Ph.D. (Leader) Andrew Perkins BS Cynthia S. Gillett, DVM, ACLAM, CPIA Ernie Prentice Ph.D. Gwenn S.F. Oki MS Jenny Kalishman DVM Kathy Wadsworth MS Mark Christensen, Professor Molly Greene MS Michael Fallon DVM, Ph.D. Paul Braunschweiger PH. D. Randall J. Nelson, Professor Susan Miller, MD Susan Silk MS Thomas Zimmerman, DVM, MPVM, DACLAM BI Choe Ph.D. Fernando Lolas MD University of Nebraska- Medical Center (Ret.) UCLA University of Minnesota University of Nebraska-Medical Center City of Hope Medical Center Washington University-St. Louis, MO UCLA Lourdes College Michigan State University Veterans Affairs University of Miami University of Memphis Weill Medical College, Cornell University Office of Laboratory Animal Welfare (OLAW) SUNY - Stony Brook Catholic University, Korea University of Chile March 28, 2008 What is the CITI Program Web based instructional program in Lab Animal Care and Use. Collaboration between the VA (Researchtraining.org) and the CITI Program. • Basic Courses – "Working with the IACUC" for Investigator, Students and staff. – “Post-Procedure Care of Mice and Rats in Research: Reducing Pain and Distress” – “Essentials for IACUC Members“ – Refresher Courses RCR 12-29-07 March 28, 2008 What is the CITI Program Web based instructional program in Lab Animal Care and Use. Collaboration between the VA (Researchtraining.org) and the CITI Program. • Specialized Model Specific Courses – – – – – – Working with Amphibians Working with Mice Working with Rats Working with Hamsters Working with Gerbils Working with Guinea Pigs – – – – – Working with Rabbits Working with Cats Working with Dogs Working With Swine Working With Nonhuman Primates • Lab Animal Research Working Group. – IACUC Chairs, IACUC Members, Coordinators, Vets, Ethicists. – Meet semi-annually RCR 12-29-07 www.citiprogram.org Establishing an Institutional Curriculum • Each institution can establish their own curriculum from the L.A.W. Content Library. • Curriculum can be customized according to: – Role in LAW research. – Species / models used. • Basic courses and refresher courses. – Reminder notifications RCR 12-29-07 New Utilities of Institutional Coordinators CITI Knowledge Base • CITI Program offers a searchable FAQ utility. • Users can submit Queries from the knowledgebase page • Topics cover many topics navigation to information for CITI administrators. • More than 150 users/month visited the page • It can be accessed directly: http://citiprogram.supportcenterpro.com/knowledgebase or in the CITI site from the contact us page: https://www.citiprogram.org/contactus.asp?language=engli sh Helpdesk Chat • • • • • • Implemented April 2010 Well received Average of 20 sections per week Chat utility permits quick answers to questions Only CITI Program during business hours. It can be accessed directly: http://citiprogram.supportcenterpro.com/liveagent/ chat/ or in the CITI site from the contact us page: https://www.citiprogram.org/contactus.asp?langu age=english Webinars How to get the most from the CITI Program • New and experienced CITI institutional administrators • Administrators of any participating institutions can take advantage of these training sessions. • Since December 2009 – Total of 55 sections with 725 participants were conducted – High level of satisfaction with the CITI webinars. • Information of how to enroll can be found at: http://citiprogram.supportcenterpro.com/knowledge base/citi-administrators/.citi-administratorwebinars.html Learner Feedback • Satisfaction Survey – Navigation – Content – Quiz question – Utility – Value Now that I have completed the course, I am more confident in my ability to advise a student or a colleague on how to apply the "3Rs" to insure that their lab animal studies are conducted to the highest ethical standards 9% 69% After completing this instruction, I now have a better understanding of how to apply the "3Rs" to insure that my lab animal studies are conducted to the highest ethical standards? 30% 9% 68% % Responders (n=1311) 25% 20% 15% 10% 5% 0% 1 2 3 Strongly disagree 4 5 6 7 8 Strongly agree 9 I intend to take a more active role in assuring that lab animal research at my institution is conducted to the highest ethical standards by pursuing professional certification, or by joining an ethics committee or Institutional Animal Care and Use Committee (IACUC). 30% 14% 58% % Responders (n=1297) 25% 20% 15% 10% 5% 0% 1 2 3 Strongly disagree 4 5 6 7 8 9 Strongly agree Responsible Conduct of Research NIH, NSF requirements Federal Register / Vol. 74, No. 160 / Thursday, August 20, 2009 / Notices http://edocket.access.gpo.gov/2009/pdf/E9-19930.pdf NSF Requirements Section 7009 of the America COMPETES Act. • RCR Training for NSF post docs and students. 1-1-10 – NSF • No specific standards, No specific content. • Will not dictate pedagogical approach. • Must document. • “Therefore, it is the responsibility of each institution to determine both the content and the delivery method for the training that will meet the institution’s particular needs for RCR training in all areas at that institution for which NSF provides support.” NIH Requirements • Training Grants – Who needs training – Topics to be included. – How instruction should be delivered. – How often. – When training should be applied. – Grant review process. CITI RCR Course Biomedical Sciences Social & Behavioral Sciences* Physical Sciences Arts & Humanities Engineering Science Administrators Supported by a contract from DHHS / ORI The Responsible Conduct of Research • Public Access vs. Subscriber access • Topics – – – – – – – – – Research Misconduct Data Acquisition and Management Conflicts of Interest Responsible Authorship Responsible Peer Review Human Subjects Protection Lab Animal Welfare Mentoring Responsible Collaborative Research • Customized courses. • Text, cases, videos and quizzes CITI RCR Courses. • CITI RCR COURSES – – – – Cover the 9 topic areas as indicated by ORI and NSF. Text, cases, video cases, quizzes. Discipline specific. Can be customized according to the needs of institution, investigator or student. • Best used as an Introduction to RCR in a programmatic approach to RCR education. • Public access availability at the CITI Program Home Page www.citiprogram.org Content Format Publication Practices and Responsible Authorship • Introductory video cases - 10 – 10, 3 minute video cases. – 3 new “introductory” video case studies for the SBR investigators and students. • Misconduct • Data Acquisition • Conflicts of Interest Lab Animal Welfare Other new projects Course in Bio-safety and Bio-security Bio-Safety and Bio-Security Module # 19 10 2 11 3 12 4 13 5 14 6 15 7 16 8 Module Title Overview Risk Management - Emergency Procedures Lab OSHA Associated Blood-borne Infections Pathogens Risk NIH Recombinant Assessment DNA Guidelines Medical Human Gene Surveillance Transfer Risk Select Management Agents, Biological – WorkSecurity, Practices and Bioterrorism Risk Shipping Management and Transport – Personal of Regulated Protective Bio-Materials Equipment Risk Animal Management Biosafety – Engineering Controls Risk Nanotechnology Managementand – Lab Safe Design Practices Summary • The active promotion of integrity in the research enterprise is essential to maintain the Public Trust. – Without the Public Trust there can be now research • Management of conflicts of interest between – Investigators, the institution, and the subjects is crucial to well run research enterprise “Integrity” by Joris Plu 2005 Summary • Promoting Integrity is everyone’s responsibility. – Leads to good animal care – Leads to good science. – Compliance • The Responsible Conduct of Research is beyond simply being compliant with Federal regulations. It is just The “right thing to do”. “New Integrity” by Artibella Avanti CITI Support Staff Absent: Richard Sprince and 1 TBN content specialist “An Experiment on a Bird in The Air Pump “ by Joseph Wright (1734-1797) British National Gallery- London Joseph Wright (September 3, 1734 - August 29, 1797),