CHEM 120 Lab Schedule
advertisement

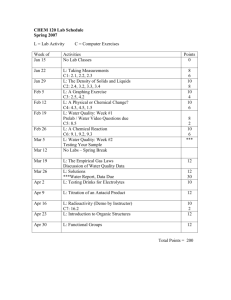
Instructor Information: Instructor name: Megan Sprague Email Address: Megan.Sprague@heartland.edu Laboratory Outline: The laboratory component of this class will consist of multiple computer-based problem sets as well as traditional lab exercises. The focus of the computer exercises is to work on the basic skills required to be proficient in Chemistry. The traditional lab experiments are designed to be both fun and reinforce the current course content. Since the lab portion of the class satisfies a graduation requirement, the lab exercises are mandatory for all students. Anyone who misses more than two lab periods can fail the entire course (usually, with each additional lab period beyond two missed, the grade is lowered by one letter grade each time). The laboratory time is a 2-hour time block please expect that each lab period will meet for the allotted period. Anyone leaving early, before completion of the activity, without permission will not receive full credit for that lab period. Also, students arriving late will be counted as a one-half absence. Lab Materials: Fundamentals of Chemistry Lab Manual, by Muench (Kinko’s Publishing) Safety Glasses (your own or the ones supplied by HCC) Scientific Calculator Lab Content: Each week will begin with the students turning in their completed pre-lab assignment. Then, I will proceed with a brief explanation of the lab procedures for that particular week. Once all of that has been completed, you will proceed with that week's lab exercise to its finality. All work, including data pages and questions, are to be turned in before leaving. In the beginning, you will also have computer assignments to do as well as a lab exercise. I would suggest that you do the computer exercises after you have completed your lab exercise. The computer exercises can be repeated to improve upon your scores both that week and in subsequent weeks afterwards as time permits. Lab Format: Most of the labs – including the computer exercises – are designed to be done as group assignments. In general, groups of two students work best. The instructor may let you choose your own group or he may assign them. Regardless, you are expected to contribute, as well as participate in, each of the activities with your group. Failure to contribute / participate will result in: first a warning to the student, then no credit for each lab session the student continues to shows a lack of involvement. If any conflicts develop between individuals, then the instructor may reassign groups. Additionally, if you have any personal conflicts with an individual in your group and would like to be reassigned, then please alert your instructor to the problem. Grading: Grades will be determined by a combination of lab work and computer-based activities. The point values of each lab and computer activity are found on the lab schedule. The total point value is equal to 200 points or approximately 20% of your overall grade. Lab Schedule: Chem120 Spring 2012 L = Lab Activity C = Computer Exercises Week of Activities Jan 16 No Labs Jan 23 Jan 30 Feb 6 Feb 13 Feb 20 Feb 27 Mar 5 Mar 12 Mar 19 Mar 26 Apr 2 Apr 9 Apr 16 Apr 23 Apr 30 May 7 Points 0 L: Taking Measurements C1: 2.1, 2.2, 2.3 L: The Density of Solids and Liquids C2: 2.4, 3.2, 3.3, 3.4 L: A Physical or Chemical Change? C3: 2.5, 4.2, 1.5 L: Testing Drinks for Electrolytes C5: 4.3, 4.5, 8.5 L: A Chemical Reaction C6: 9.1, 9.2, 9.3 L: Water Quality: Week #1 Prelab / Water Video Questions due L: Water Quality: Week #2 Testing Your Sample Spring Break – No Labs 8 6 10 8 10 6 10 6 10 6 8 L: The Empirical Gas Laws Discussion of Water Quality Data L: Solubility Limit versus Temperature 12 L: Titration of an Antacid Product Water Report, Data Due# L: Radioactivity (Demo by Instructor) C7: 16.2 L: Introduction to Organic Structures 10 30 10 2 12 L: The Biodiesel Project: Week 1 Prelab and Week 1 Data L: The Biodiesel Project: Week 2 6 # 10 20 Snow Day / Make-Up (Will be used in case of winter weather cancellation) Total Points = 200