Nucleotides and Nucleic acids
advertisement

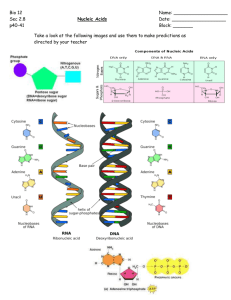
Welcome to class of Nucleotides and Nucleic acids Dr. Meera kaur Learning objectives • To understand – the names and one-letter symbols of the five major nitrogen bases found in nucleic acids – two differences between the molecular composition of DNA and RNA – the structure of DNA – the process of replication, transcription and translation – Concept of gene mutation and molecular disease – Concept of recombinant DNA technology Introduction to nucleic acids… • In 1869, Johannes Miescher of the University of Germany carried out the research on elemental analysis of the nucleus. He studied cell containing large nuclei and little cytoplasm (white blood cells). He collected white cells from pus, washed from bandages of infected surgical patients. When he extracted the nuclei with basic solution and acidified the alkaline extract, he got a precipitate of stringy material that he analyzed further. • His careful elemental analysis gave carbon, nitrogen and phosphorus in proportions that were unlike of those of carbohydrates, lipids or protein. Miescher named his new materials nuclein to reflect its nuclear origin. • Today, we called these compound the nucleic acids. Introduction to nucleic acids • Miescher made little progress in learning about nucleic acids, either their functions or structure. But other scientists eventually learned that there are two types of nucleic acids: deoxyribonucleic acid (DNA) and ribonucleic acids (RNA). Structure of Nucleic acids: - Nucleotides are the fundamental building blocks of nucleic acids. - DNA and RNA molecules are polynucleotides; i.e., polymers composed of many repeating units of nucleotides. - Each nucleotide consists a sugar unit, a nitrogen base and a phosphate group attached to the sugar unit. The combination of a base and sugar units makes a nucleosides. Adding a phosphate group to the sugar unit of a nucleoside makes nucleotide. Structure of nucleic acids BASE + SUGAR UNIT BASE------SUGAR + PHOSPHATE A nucleoside BASE---SUGAR A nucleoside BASE— SUGAR---PHOAPHATE A nucleotides Structure of the sugar units • The sugar unit of the nucleotides strung together to make RNA is -D- ribose -- hence the name ribonucleic acid • The sugar unit of DNA is -D-2-deoxyribose— hence the name deoxyribonucleic acid Structure of the base units • Four different nitrogen bases (heterocyclic amines) are found in DNA. • Two of these bases - adenine (A) and guanine (G) - are derivatives of purine. They are called purine bases. • The other two cytosine (C) and thymine (T) are derivatives of pyrimidine. They are called pyrimidine bases. • Except for thymine, the same bases are found in RNA. Instead of thymine, uracil — a pyrimidine base is found in RNA. Basic structure of nucleotide (nitrogenous base, pentose, and phosphate) Carbons of the sugar ring are numbered 1, 2, 3, 4 and 5(read as “one prime”, “Two prime” and so forth) Nitrogenous bases of nucleotides The ring atoms of the nitrogen bases are numbered as 1, 2, 3, 4, 5 and so forth Major purine and pyrimidine bases of nucleic acids Structure of nucleosides… • The base and sugar units of nucleosides are held together by a covalent bond between the nitrogen of a purine or pyrimidine bases and the ring carbon of the sugar unit. • Ribonucleosides (nucleosides found in RNA) are similar in structure to the deoxyribonucleosides, except that ‘ribose’ rather than ‘deoxyribose’ is the sugar and uracil replaces thymine. • Purine nucleosides are formed by a covalent bond between nitrogen 9 of the base and 1 of the sugar unit. • The base and sugar of the pyrimidine nucleosides are joined by a covalent bond between nitrogen 1 of the base and carbon 1 of the sugar. Structure of nucleotides • Addition of a phosphate group to a sugar hydroxyl group forms a nucleotide. Some adenosine monophosphates Examples of nucleotides The covalent backbone structure of DNA and RNA In DNA and RNA molecules, nucleotides are linked together through phosphate ester bridge between the 3hydroxyl group of one nucleotide and the 5hydroxyl group of the next nucleotide in the chain. These bridges are called phosphodiesters. Even though two of the four oxygen attached to the phosphorus of each bridge are tied up as phosphate esters, and one is present in phosphorus—oxygen double bond, one oxygen is free to lose a proton. It is the presence of many such dissociating groups that gives DNA and RNA their highly acidic character. Shorthand structures for nucleotide sequence • DNA and RNA molecules are too complex to write in full. So biochemists often use a shorthand form. • The basis for the shorthand is the differing order of their bases. The sugar units and the phosphate groups are identical in all the nucleotides. • We can write the structure of an RNA by ignoring the sugars and the phosphodiester bridges, and by writing only the sequence of nitrogen bases. Start at the left with the end of the molecule that has a free 5end and work toward the end that has a free 3hydroxyl group U—A—G—C—U—G—C—C 5 ________________ 3 RNA dA—A—T—G—T—C—A—C 5 __________________ 3 DNA Avery-MacLeod-McCarty experiment… • 1944 • First direct evidence that DNA is the bearer of genetic information • PROBLEM: not universally accepted, since protein impurities in the DNA could have carried the message The Avery-MacLeod-McCarty Experiment… Adding heat killed virulent bacteria to a live nonvirulent strain permanently transformed the latter into lethal, virulent, encapsulated bacteria. He concluded that a transforming factor in the heat killed virulent bacteria had gained entrance into the live nonvirulent bacteria and rendered them virulent and encapsulated. Avery and his colleagues identified this transforming factor as DNA.